Exploring the endometrium one cell at a time: An interview with Dr. Roser Vento-Tormo
While the field of single cell biology has seen the power of what single cell gene expression analysis can do, researchers are increasingly pairing spatial location with transcriptomics to make even further strides in the areas of immunology, cancer, neuroscience, and others. In this feature, we’ll spotlight the work of one scientist, Dr. Roser Vento-Tormo at the Wellcome Sanger Institute, who is using single cell and spatial tools to advance our understanding of the interaction between maternal and fetal cells in the womb. Read more to find out how these paired insights into cell–cell interactions are poised to advance women’s health.
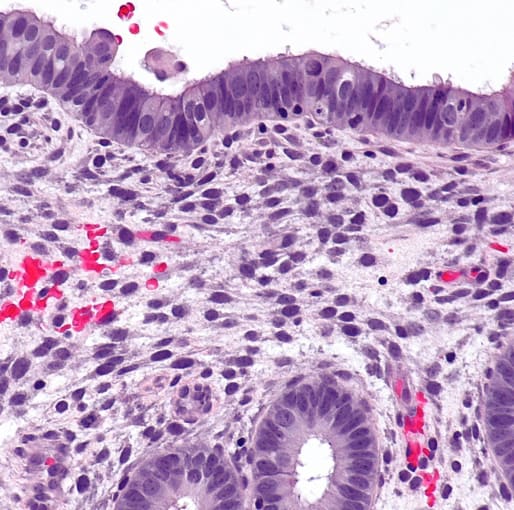
Problems within the endometrium, which is the mucosal lining of the uterus, affect the sexual and reproductive health of women worldwide. However, over the past decade, there hasn’t been much progress in studying many of these disorders, including abnormal uterine bleeding, infertility, miscarriage, pre-eclampsia, endometriosis, and endometrial cancer, partly due to fact that the endometrium is a complex, ever-changing tissue. With tools that now enable single cell resolution of different cell types within the endometrial tissue as well as how the tissue changes during the menstrual cycle and pregnancy, scientists have opened a new window into a microenvironment influenced by fluctuating levels of hormones, immune cells, and more.
This complexity is precisely what drew Roser Vento-Tormo, PhD, to the field. Dr. Vento-Tormo earned her doctorate in the lab of Esteban Ballestar, PhD, in Barcelona, where she studied the influence of cytokines on innate immune cell differentiation. She completed postdoctoral studies with Sarah Teichmann, PhD, at the Wellcome Sanger Institute, working on tissue resident immune responses with a focus on the maternal–fetal interface during pregnancy.
With the help of Dr. Teichmann—a leader of the Human Cell Atlas initiative, which aims to map every cell type in the human body—Dr. Vento-Tormo created the first single cell map of the maternal–fetal interface during early pregnancy. She also uncovered new cell states with a role in controlling inflammation in the womb. As a postdoc, Dr. Vento-Tormo designed the first version of CellPhoneDB, a database of ligands, receptors, and their interactions, and used the software to study single cell transcriptomics data.

“During my time in the Teichmann lab, I was exposed to the revolution of single cell genomics and the beginnings of the Human Cell Atlas project,” Dr. Vento-Tormo says, which influenced her decision to start her own lab at the Wellcome Sanger Institute in 2019. She and her team work on genomics and computational tools to better understand the influence of the cellular microenvironment on cell fate and function.
For immunologists, being able to study the placental–uterine interface during pregnancy is a goldmine. “Here at this interface, maternal immune cells from the uterus and fetal trophoblast cells from the placenta interact with each other peacefully,” she says. “I had the opportunity to work closely with Ashley Moffett, MD, from Cambridge University, who is a worldwide expert on the uterine immune microenvironment. During my long conversations with Professor Moffett, I realized how little we know about the uterus and its cellular and molecular changes during the menstrual cycle, pregnancy, and age. This has led to drastic consequences for women’s health, infertility, and pregnancy disorders. For example, 10% of women of reproductive age have endometriosis, but non-invasive diagnostic tools and efficient treatments are still missing.”
Exposing endometrial heterogeneity with single cell and spatial technologies
To that end, Dr. Vento-Tormo is combining single cell and spatial technologies. She and her team are using 10x Genomics single cell and spatial solutions to create single cell and spatial reference maps of the human uterus and endometrium. From these, they’re learning more about how the two tissues interact during a typical menstrual cycle as well as the dysfunctional states leading to diseases like endometriosis. Preliminary results are available in her recent bioRxiv pre-print paper, titled “Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro.”
“In morphologically complex tissues such as the uterus, the location of a cell has an enormous influence on its activity and function,” she says. “For example, epithelial cells in the glands secrete molecules that provide nutrition to the embryo while epithelial cells in the lumen are key for fetal implantation.”
Multidimensional approaches will help improve our understanding of endometrial tissue complexity. “Spatial transcriptomics is allowing us to characterize the function and molecular regulation of the new epithelial cell states [that] we identify by single cell transcriptomics,” she says. “Our preliminary results reveal two new progenitors with unique spatial coordinates that have the ability to differentiate into lumenal and glandular cells.”
Dr. Vento-Tormo and colleagues are also looking at the mechanisms behind signaling pathways in lumenal and glandular microenvironments. “We’re now using endometrium organoids to dissect how NOTCH and WNT pathways interact with ovarian hormones during endometrial regeneration and differentiation.”
Expanding the cell–cell communication database with spatial transcriptomics
Finally, they are further developing CellPhoneDB v3.0 to enable measurement of cell–cell communication that now includes the spatial coordinates of cells.
“Tissue architecture underlies cellular communication and function,” Dr Vento-Tormo says. “During my postdoc, I predicted communication between maternal immune and fetal trophoblast cells at the uterine–placental interface considering the expression of ligands and receptors. With the new spatial transcriptomics methods, we can now improve predictions by considering both the expression of ligands and receptors, and the proximity of communicating cells. This is the basis of the new version of CellPhoneDB that my team has recently developed.”
It’s apparent that these mechanistic insights could lead to the development of treatments for a range of common endometrial disorders. It seems the field is on the cusp of realizing the combinatory power of single cell and spatial methods for scientific and translational research.
“Combining multiple technologies at the single cell level will allow us to better understand how cells respond to complex signals in tissues,” she says. “Describing the principles underlying cellular regulation in its native environment will inform the development and improvement of current in vitro models with important clinical implications.
“In addition, the revolution of genomics technologies means that we can apply single cell and spatial technologies to hundreds of donors in parallel. We are starting to identify molecular and cellular changes in multiple individuals, which is key [to] targeted therapies and personalized medicine. It is an exciting time to be in the genomics field!”
To learn more about Dr. Vento-Tormo’s research using single cell and spatial tools to map the human endometrium, register for her upcoming webinar.
This article contains a discussion of research conducted by members of the Cellular Genetics group led by Dr. Roser Vento-Tormo at Wellcome Sanger Institute. View and opinions do not constitute endorsement or promotion of 10x Genomics, Inc. or any of its products.
