Seeing novel targets for glaucoma treatments with single cell transcriptomics
In honor of National Glaucoma Awareness Month in January, we highlight a publication that probes deeper into the signaling pathways that may drive the development of glaucoma, a leading cause of blindness. In this study, led by Benjamin Thomson, PhD, a research assistant professor at Northwestern University Feinberg School of Medicine, single cell transcriptomic analysis of a glaucoma mouse model was used to identify distinct cell populations and key signaling pathways that may be involved in disease progression, offering novel targets for glaucoma treatments.
Over 60 million people worldwide are affected by glaucoma, a progressive neurodegenerative disease that leads to increased pressure in the eye, damaging the optic nerve and causing blindness (1). Unfortunately, because there are no symptoms, many people will never know they have glaucoma until it has significantly progressed. Current therapies, including prostaglandin analogs and beta blockers administered with eye drops, do succeed in lowering intraocular pressure (IOP) and, thereby, slowing progression. However, these treatments are not always effective for every patient, making the need for new, better therapies imperative.
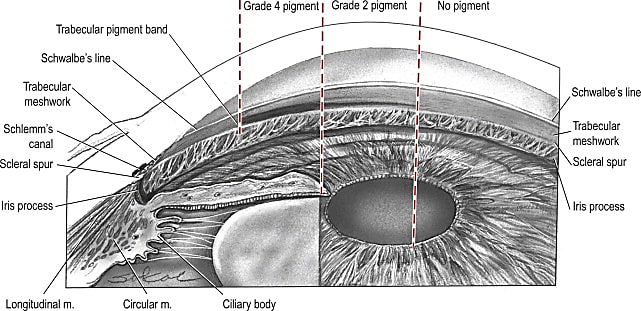
In healthy eyes, a watery fluid called the aqueous humor provides nutrients and is constantly being replenished then drained through a network of tissues and cells. The trabecular meshwork (TM) is a tissue located near the base of the cornea that drains the fluid of the eye into a set of tubes called Schlemm's canal (SC). When adequate outflow becomes blocked at the TM, fluid builds up and increases pressure in the eye. This can cause damage to the optic nerve and vision loss.
While most types of glaucoma occur in adults over 40 years old, primary congenital glaucoma (PCG) affects children between birth and 3 years (2) and is driven by developmental defects in the trabecular meshwork and Schlemm's canal. Loss-of-function variants in TEK (tunica interna endothelial cell kinase) or its primary ligand, ANGPT1 (angiopoietin 1), are linked to primary congenital glaucoma in children (3), while ANGPT1 is associated with primary open-angle glaucoma in adults (4).
TM–SC signaling via ANGPT1 and SVEP1, a protein necessary for lymphatic development and valve formation, is important for the development of Schlemm's canal and homeostasis of intraocular pressure (5), but numerous other pathways, many of which are not yet described, are also involved in this signaling regulation. To help uncover additional pathways that might be important in regulating fluid outflow and, therefore, offer additional therapeutic targets for treating glaucoma, Dr. Thomson and his team performed single cell transcriptomic analysis on wild-type and glaucomatous Angpt1-deficient eyes (using an Angpt1-knockout mouse model). They identified TM- and SC-specific molecules and signaling networks that offer evidence of TM–SC crosstalk being essential for regulating IOP homeostasis (6).
Single cell readouts provide clues to understanding TM–SC crosstalk
In the first part of their study (6), Dr. Thomson and his team performed functional assays to show that not only is Angpt1 expressed by neural crest–derived cells, including trabecular meshwork cells, but that deleting Angpt1 or Svep1 from the TM causes primary congenital glaucoma in mice with severe defects in the Schlemm's canal.
From their transcriptomic data, they identified 25 different cell types and showed that similar cell populations were present in both the control and Angpt1-deficient samples. They identified 11 groups of TM cells and, as expected, observed few or no Angpt1-expressing cells in Angpt1-deficient samples.
They identified four endothelial cell populations in which the SC endothelial cells expressed specific markers distinguishing them from lymphatic endothelial cells. While SC endothelial cells may share similarities to the lymphatic phenotype, their transcriptome was observed to be more closely related to blood vascular endothelial cells. Additionally, they saw very few SC endothelial cells in Angpt1-deficient mice compared to controls.
TM–SC crosstalk and the ANGPT–TEK axis are promising glaucoma therapeutic targets
Comparing their cell-type markers to curated lists of glaucoma-associated genes in search of novel targets, Dr. Thomson and his team were able to identify 21 genes in Schlemm's canal endothelial cells, including Cav1, Cav2, Tgfbr3, Foxc1, Tek, Flt1, Kdr, and Flt4, and 30 genes in trabecular meshwork cell clusters, including Foxc1, Myoc, Angpt1, Svep1, and Vegfa.
Finally, functional work showed that an ANGPT1-mimetic could spur the development of SC in wild-type and Angpt1-deficient mice as well as lower intraocular pressure in healthy adult mice, making targeting the entire pathway (and not just individual genes) promising. In all, the results of this study show that there is a large group of potentially new therapeutic glaucoma signaling molecules regulating the development and function of the outflow pathway of the eye that can be targeted for improved IOP-reducing therapies.
References:
- https://www.glaucoma.org/news/glaucoma-awareness-month.php
- https://www.webmd.com/eye-health/primary-congenital-glaucoma
- Thomson BR, et al. Angiopoietin-1 is required for Schlemm’s canal development in mice and humans. J Clin Invest 127: 4421–4436 (2017). doi: 10.1172/JCI95545.
- MacGregor S, et al. Genome-wide association study of intraocular pressure uncovers new pathways to glaucoma. Nat Genet 50: 1067–1071 (2018). doi: 10.1038/s41588-018-0176-y.
- Bartlett CS, Jeansson M & Quaggin SE. Vascular growth factors and glomerular disease. Annu Rev Physiol 78: 437–461 (2016). doi: 10.1146/annurev-physiol-021115-105412.
- Thomson BR, et al. Cellular crosstalk regulates the aqueous humor outflow pathway and provides new targets for glaucoma therapies. Nat Commun 12: 6072 (2021). doi: 10.1038/s41467-021-26346-0.
