Single Cell Gene Expression Aiding Diabetes Research
Guest Author: Jennifer MacArthur
One of the main jobs of the pancreas is to sense and regulate levels of glucose in the blood. Pancreatic islets are clusters of hormone-producing cell types—such as alpha cells that make glucagon, and beta cells that make insulin. These cells respond to blood glucose concentration and signal the body to raise or lower blood sugar as needed. People with type 1 diabetes (an autoimmune disease that destroys the beta cells) rely on insulin therapy to manage their blood sugar levels. Despite advances with insulin analogs, pumps, and continuous glucose monitors, nothing is as effective at regulating blood sugar as a fully functioning pancreas.
Because November is National Diabetes Month, we wanted to highlight some of the exciting diabetes research published this year, and how 10x Genomics technology played a part in these discoveries. One paper was focused on how to potentially treat diabetes by inducing non-beta cells to secrete insulin. Another group looked at how beta cells get targeted by the immune system in the first place and found promising evidence for ways to prevent the disease.
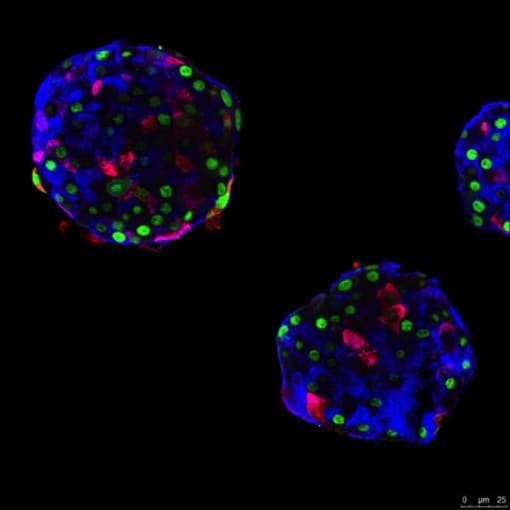
With the goal of replacing dysfunctional beta cells in type 1 diabetes, a laboratory at the University of Geneva in Switzerland successfully reprogrammed human pancreatic alpha cells to sense glucose and make insulin. These beta-like cells corrected diabetes in mice, and the effect was sustained. Six months after transplantation, the modified alpha cells continued to secrete human insulin in response to high glucose. Notably, the insulin-producing alpha cells maintained expression of alpha-cell markers (1).
“They have a hybrid personality,” said senior author Pedro Herrera in a statement. "We found that modified alpha cells triggered a weaker immune response, and therefore might be less likely to be destroyed than native beta cells."
To map the transition from glucagon to insulin secretion at single cell resolution, these researchers used single cell gene expression profiling. This method helped them find several genes not detected by bulk RNA-seq, and pinpoint potential target pathways to induce this alpha cell reprogramming in situ (1).
"We must indeed find a way—pharmacological or by gene therapy—to stimulate this change of identity in the cells concerned within the patient's own pancreas, but without causing adverse effects on other cell types," said Herrera.
Targeted elimination of senescent beta cells prevents type 1 diabetes
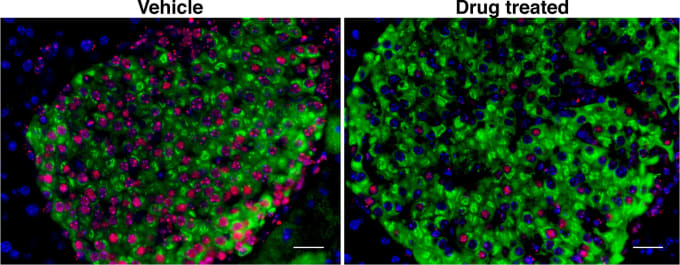
It’s usually thought that pancreatic beta cells are passive victims of immune cell attack in type 1 diabetes. However, researchers at the University of California, San Francisco wanted to examine that assumption. They used single cell gene expression to look with intricate detail at early disease development in non-obese diabetic (NOD) mice. Transcriptome analysis revealed a beta cell subpopulation with a senescence stress response phenotype. These senescent beta cells secrete proinflammatory molecules that damage nearby cells and may be the trigger for autoimmunity. Beta cell samples from humans with early signs of type 1 diabetes showed these senescent signatures too. Taking advantage of an FDA-approved drug that specifically eradicates senescent cells, the research team tried it as a preventative treatment in NOD mice, and the team’s data indicates that it worked (2).
“This is a paradigm shift for type 1 diabetes therapy,” Anil Bhushan, who led the study, said in a statement. “These data suggest the problem may not be an immune system gone awry. Instead, perhaps therapies should find a way to do the job the immune system is failing to do: clear the senescent cells early on.”
This type of research is part of the mission of 10x Genomics. We are building the tools that will help scientists interrogate, understand and master biology. We look forward to more discoveries enabled by our solutions that will advance human health.
To learn more about solutions for immunology research, please visit 10xgenomics.com/immunology.
References
- K. Furuyama, S. Chera, L. van Gurp et al., Diabetes relief in mice by glucose-sensing insulin-secreting human α-cells. Nature. 567, 7746 (2019).
- P. Thompson, A. Shah, V. Ntranos et al. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metabolism. 29,4 (2019).
