Complementary single cell and spatial platforms construct a spatially resolved gene expression atlas of maize embryos
Cornell University scientists have discovered an “ancient, convergent mechanism” linking the development of embryonic organs in maize, Arabidopsis (the famous plant model organism), and moss (1). This leap in our understanding of evolutionarily maintained gene expression networks across plant groups was enabled by a single cell spatial analysis of maize embryos with Chromium, Visium, and Xenium—the first study of its kind to combine all three high-resolution single cell and spatial transcriptomics platforms in plant tissue.
Defining terms
We’re all experts in something, but we’re not all experts in taxonomy, evolutionary biology, or plant embryogenesis! In order to understand the content in the rest of this article, we thought some of the following terms would be important to define:
Phylogenesis: The evolutionary development and diversification of a species or group of organisms, or of a particular feature of an organism.
Phylogeny: A diagrammatic hypothesis about the history of the evolutionary relationships of a group of organisms.
Kingdom: The highest category in taxonomic classification.
Phylum: A principal taxonomic category that ranks above class and below kingdom.
Clade: A group of organisms believed to have evolved from a common ancestor.
Phylostrata: The set of organisms which are not yet present in a previous clade, but appear in the new clade, and then are left behind when the next clade begins (2).
Sequence homology: The biological homology between DNA, RNA, or protein sequences, defined in terms of shared ancestry in the evolutionary history of life.
Ortholog: Homologous sequences are orthologous if they are inferred to be descended from the same ancestral sequence separated by a speciation event: when a species diverges into two separate species, the copies of a single gene in the two resulting species are said to be orthologous. Orthologs, or orthologous genes, are genes in different species that originated by vertical descent from a single gene of the last common ancestor.
Cotyledon: An embryonic leaf in seed-bearing plants, one or more of which are the first leaves to appear from a germinating seed.
Monocot: Short for monocotyledon, a flowering plant with an embryo that bears a single cotyledon (seed leaf).
Dicot: Short for dicotyledon, a flowering plant with an embryo that bears two cotyledons (seed leaves).
Credit: Oxford Dictionary, Wikipedia.
Single cell and spatial transcriptomics map maize embryonic development
Did you know maize, commonly referred to as corn, is a grass? A delicious grass beloved by many. It also happens to be a go-to model organism for a number of plant development studies, and the star of a recent bioRxiv preprint (1) from a Cornell University research team.
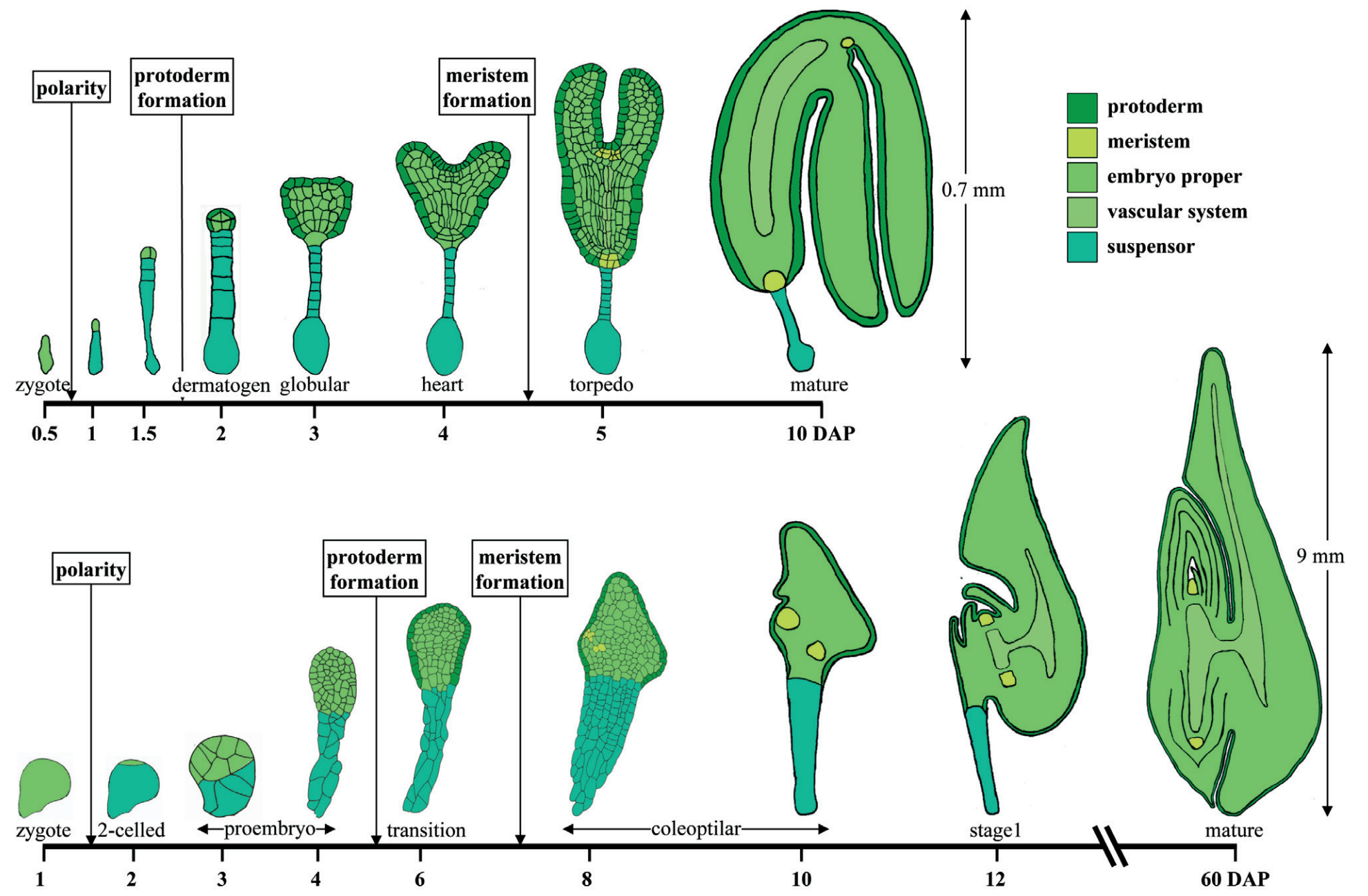
Just like humans and animals, plants have an embryonic stage where the fertilized seed begins to change dramatically—cells divide, causing the embryo to enlarge, tissue layers begin to form, and new organs branch out, establishing the structures that will go on to become roots, stems, and leaves.
But what happens at a molecular level (and where) during these big changes? What gene expression programs drive this almost miraculous emergence of complex organs, and eventually a mature plant, from a tiny seed? And, given the common patterns of embryogenesis seen across species and larger plant groups, how might these gene programs have been conserved over the evolutionary ages?
To answer these questions, a research team from Cornell University leveraged Chromium Single Cell Gene Expression (recently renamed Universal 3’ Gene Expression) to study the transcriptional profiles of the cells that compose Stage 1 maize embryos. Embryos in this stage contain all of the lateral organ types, making it a key transition point to uncover the underlying cellular and molecular mechanisms driving organ formation. They profiled 11,467 protoplasts (plant cells without the cell wall) from over 400 Stage 1 embryos, identifying 23 cell clusters.
Embryogenesis is a fundamentally spatial process—it is essential to establish and maintain spatial and functional differences in the developing embryo to properly organize the tissues, guide cell differentiation, and ensure the correct body plan. Therefore, the tissue of origin for each of these 23 cell clusters was an essential piece of information for accurately identifying the cell type. The team turned to Visium Spatial Gene Expression (v1 assay) to analyze eight Stage 1 embryo sections, which offered a spatially resolved readout of the whole transcriptome to define cell clusters in morphological context. Visium data identified nine cell clusters, further categorized into four regions aligned to major organs or developmental structures, including the shoot–root axis or the large, internal scutellum. By integrating Visium data with single cell RNA-seq from Stage 1 embryos, the authors mapped gene expression to precise locations in the embryos and confirmed that 14 of the 23 single cell clusters originated from the scutellum.
Resolving the remaining clusters originating from the smallest structures in the embryo, including the leaf, required a higher resolution spatial assay, leading the team to use Xenium In Situ analysis. They developed a custom maize panel consisting of 99 genes, including differentially expressed regulatory genes identified from their single cell dataset. Then, they profiled maize embryo sections from three successive developmental stages: transition-staged embryos forming the shoot apical meristem and scutellum, coleoptilar-staged embryos, and the classic Stage 1 embryos that showed growth of the first leaf. This analysis revealed spatiotemporal gene expression patterns at single cell resolution of several key regulatory genes, such as the indeterminacy marker KNOTTED1 (KN1); CUP-SHAPED COTYLEDON2 (CUC2), which marks developmental boundaries; and a transcription factor for mediolateral expansion, NARROW SHEATH1 (NS1).
Leveraging Xenium, the team observed some gene expression patterns that were previously undescribed. For example, they saw that NS1 transcripts accumulated in the initiating scutellum during the transition stage. CUC2 transcripts were detected between expression domains of KN1 and NS1 during scutellum initiation, as well as between the shoot apical meristem and lateral organs. DROOPING LEAF1 (DRL1), a gene involved in regulating leaf architecture, was not detected in the coleoptile or scutellum at all across the embryonic stages. Finally, the team observed transcript accumulation of two genes, BOPa and YAB14, in the scutellum and coleoptile. These genes are known regulators of polarity, organ development, and tissue differentiation in Arabidopsis embryogenesis, and, therefore, may play a similar role in maize; moreover, their expression patterns may suggest the scutellum and coleoptile are components of a single grass cotyledon.
Maize is a crucial crop plant that has a long history of cultivation and, subsequently, genetic research. These novel findings add to the established data about the gene expression patterns in complex structures within the maize embryo, ultimately enriching our understanding of maize organ evolution.
Comparing embryonic gene networks across plant groups points to gene age
The gene expression programs that guide organ development in early maize embryos have an evolutionary history—they arose at a specific point in time in the ancient past. That event drove the divergence of all embryonic land plants from non-embryonic fungi and algae (3).
Yet even embryonic plants can be very different from each other. They may come from entirely different phyla, as is the case with non-vascular, spore-producing mosses and the vascular seed plant maize. Or, they can differ morphologically, even in the earliest stages of embryogenesis. For example, maize is a monocot, while the vascular seed plant Arabidopsis is a dicot. How might embryonic plants that have evolved from the same common ancestor, yet diverged in these ways, compare in terms of their organ-initiating gene programs? What has been conserved and what has changed over evolutionary time?
To untangle these mysteries, the research team established a core group of 130 genes that were significantly upregulated during initiation of maize embryonic organs (1). Then, they cross-referenced this maize embryonic-organ-initiation gene network with established transcriptomic datasets derived from the embryonic lateral organs of Arabidopsis thaliana, a flowering plant from the mustard family (4) and a well-studied model organism. Out of the 130 core genes from the maize gene network, the team found 49 Arabidopsis gene orthologs expressed during embryonic development. Importantly, these orthologs also had stage-specific patterns of expression and transcript accumulation in Arabidopsis organs—for example, maize transcripts that accumulated in the expanding Stage 1 scutellum correlated with the expression of orthologous Arabidopsis genes during the later stages of cotyledon growth (called the late-torpedo stage). This provided evidence that despite the morphological differences between the monocot maize and the dicot Arabidopsis embryos, they share homologous gene regulatory networks during the development of certain embryonic lateral organs.
The research team compared the same maize embryonic-organ-initiation gene network with the embryonic gene expression signatures from the moss Physcomitrium patens, which represents a distinct plant phylum. In contrast to maize’s complex organ-initiating gene network, moss bears a simpler, ancestral gene network focused on basic polarity and differentiation, reflecting its early evolutionary position among land plants. Notably, moss transcripts during mid-embryogenesis were distinct from the gene networks expressed in Arabidopsis and maize at the same stage. Correlation between the gene networks increased, however, in the early and late stages of embryogenesis, which is a similar, inverse pattern observed in across-phylum comparisons of animal embryos.
To finally understand when these conserved gene networks may have arisen in the course of evolutionary history, the team performed a statistical analysis to compare the 130 maize transcripts with those observed from over 350 plant species organized into 13 distinct groups, or phylostrata, representing the window of time when the unique gene composition of these organisms arose. This revealed that less than 10% of the maize embryonic-organ-initiation gene network transcripts arose in the most recent phylostrata. Rather, the majority of maize transcripts appeared in three of the oldest phylostrata, including the Bryophyte phylostratum, representing plants that first had embryos; additionally, 12 transcripts were found in the algal phylostratum, representing plants that lack embryos.
This suggests that the maize embryonic gene network consists of some very old, conserved genes that straddle tremendous evolutionary changes—confirming once again, the enduring place of corn in evolutionary history, modern agriculture, and in our lives today.
Explore some of the products used for this study:
- Chromium Single Cell Gene Expression (recently renamed Universal 3’ Gene Expression)
- Visium Spatial Gene Expression
- Xenium In Situ
- Xenium In Situ Panels
References:
- Wu H, et al. Multiplexed transcriptomic analyses of the plant embryonic hourglass. bioRxiv (2024). doi: 10.1101/2024.04.04.588207
- Litman T and Stein W. Obtaining estimates for the ages of all the protein-coding genes and most of the ontology-identified noncoding genes of the human genome, assigned to 19 phylostrata. Semin Oncol 46: 3–9 (2019). doi: 10.1053/j.seminoncol.2018.11.002
- Donoghue P, et al. The evolutionary emergence of land plants. Curr Biol 31: R1281–R1298 (2021). doi: 10.1016/j.cub.2021.07.038
- Arabidopsis: The Model Plant. https://www.nsf.gov/pubs/2002/bio0202/model.htm#content
About the author:

