Fixing limitations: An scRNA-seq protocol for fixed tissues
“We want to be able to store these tumor samples and run them when we know which ones are the most interesting.” –Translational researcher
“A lot of times, samples don’t even arrive until 4 or 5 PM. Those projects are really hard on our staff.” –Director of a Core Lab
“We have to say ‘no’ to so many interesting samples that we would love to analyze because the sample management is too cumbersome.” –Researcher from a large pharmaceutical company
As these customer quotes illustrate, sometimes the hardest part of a single cell experiment is working with the logistical or handling limitations of your samples. Fresh tissue offers a wealth of information unavailable in other sample types, but the need to process it quickly can sometimes be at odds with more complex experimental designs. Perhaps your multi-site project isn’t feasible due to transportation and logistics concerns. Maybe your cell type(s) of interest are fragile and won’t survive dissociation or storage, or you’d like the option to batch and multiplex samples to reduce costs and increase efficiency.
Those limitations are why our team developed this single cell RNA sequencing (scRNA-seq) protocol for PFA-fixed tissues. In this blog you’ll get a quick snapshot of how it offers you access to more samples, lets you process them on your schedule, retains your sample quality throughout storage and transport, and provides the greatest sensitivity of any commercial fixed RNA profiling protocol. Or, if you’re ready to jump right in, we have a direct link to the protocol and a webinar that goes into greater depth (and also offers a preview of FFPE tissue compatibility).
Expanding your range of sample access
Collecting samples is only part of the challenge—you have to be able to access them. Large-scale projects such as longitudinal or multi-cohort studies provide more samples but logistics—remote collection sites, long transportation times, etc.—present challenges. Similarly, biobanks can have thousands of fixed archival samples, but using them in scRNA-seq can be problematic.
Using the new fixed RNA protocol, however, expands your sample access by addressing these limitations. The expansion of sample compatibility to include PFA-fixed tissues and cells provides access to a greater diversity of tissue collection strategies. Similarly, fixing samples at the point of collection allows extended storage (days to months) that affords the option of centralized (rather than on-site) processing and simplifies sample transport.
As in shipping and delivery, logistics are critical to research success, so make sure yours are solid!
Giving you more time to think your samples through
Living tissue doesn’t wait: if you’re collecting rare samples or doing time point analyses, you (and your lab staff) often don’t get to decide when, how many, or which samples you process.
Fixed samples, however, let you collect multiple samples, fix, store them at –20° or –80° C, and then take your time deciding which samples you want to sequence. This approach also enables you to batch and multiplex your samples, saving your lab money and maximizing efficiency without impacting sample quality.
Practically, this means you can process samples on your own time and start (or finish) your day when you feel like it, rather than when your samples demand it.
Keeping sample quality on lockdown
Preserving not just RNA representation, but tissue and cellular heterogeneity, is critical for scRNA-seq. Accomplishing this, however, can be a challenge in and of itself due to fragile cell types, high RNase activity, and rapid biological responses.
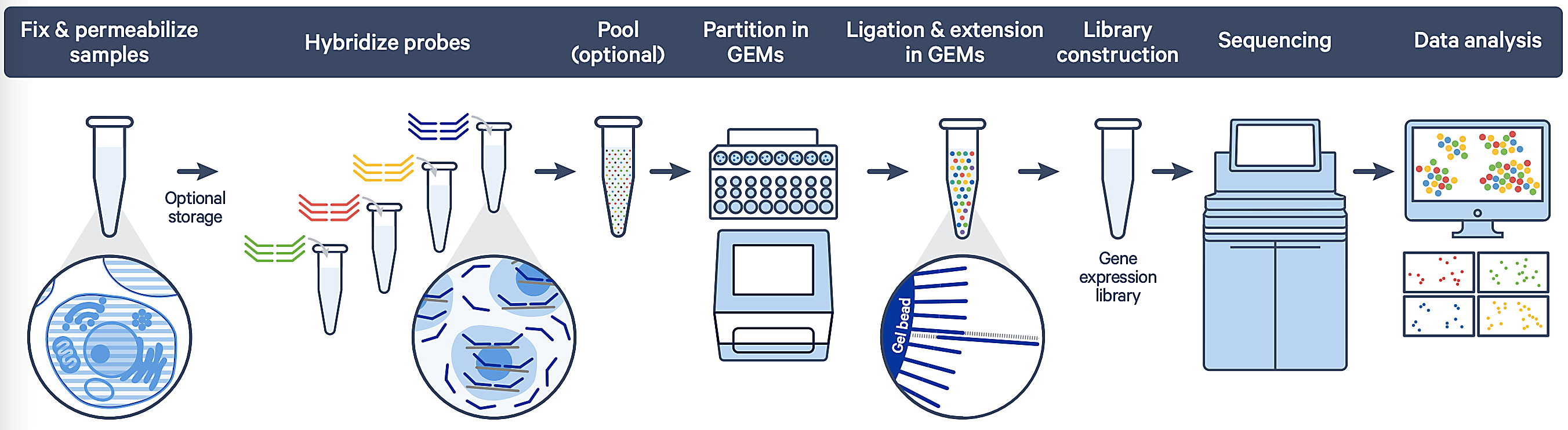
The fixed RNA protocol enables you to “lock in” the biological state of your samples at the point of collection with a fixative and subsequently capture fragmented RNA (with the option of cell surface protein analysis for multiomics) using barcoded probe templates (Figure 1). This allows you to capture rapid/small changes on shorter time scales, preserve rare and/or sensitive cell types that may not survive storage and dissociation, inactivate RNases in high-RNase tissues, and retain overall sample quality.
Sample quality is the foundation of your experiments—so make sure it’s a strong one!
Creating new possibilities for your lab
“If we could fix samples on-site, that would allow them to be collected and transported to off-site laboratories without sacrificing integrity or data quality. That would create new possibilities!” –Manager from a CRO
While not a replacement for experiments with fresh and frozen tissues, this fixed RNA protocol creates new possibilities and can open up novel avenues of inquiry for your lab. Now that you’ve seen a brief overview of the challenges it can help you overcome, take the next step: read the protocol or take a deeper dive (and preview FFPE compatibility) in our recent webinar.
