Improving response to checkpoint blockade: Single cell transcriptomics reveals new T-cell targets
Lung cancer leads the way for deaths caused by cancer in both men and women. In fact, every year, more people die of lung cancer than of colon, breast, and prostate cancers combined (1). Although several treatments and therapies approved in the last 15 years offer a marked improvement in survival rates—namely, targeted therapies and immune checkpoint inhibitors—scientists and clinicians are still puzzled as to why, in some patients, some therapies either stop working after a short period of time or don’t work at all (2). In this blog post honoring Lung Cancer Awareness Month in November, we take a look at one publication that used single cell technologies to tease out subpopulations of T cells within a mouse model of lung adenocarcinoma, discovering a type of dendritic cell that may be targeted to make immunotherapy more effective in the future.
Tumors are smart. Not only can they hijack normal cellular processes to grow and spread, but they can also acquire genetic mutations that allow them to become resistant to therapies that once worked to kill cancer cells or quell their activity. One way tumors avoid the body’s cancer-killing T cells is by activating checkpoints that cause these T cells to ignore the tumor. To beat tumors at their own game, scientists have developed antibody-based checkpoint blockade therapies, otherwise called immune checkpoint inhibitors (ICIs), that work by binding checkpoint receptor proteins so that tumors can’t access them and prevent T cells from doing their job.
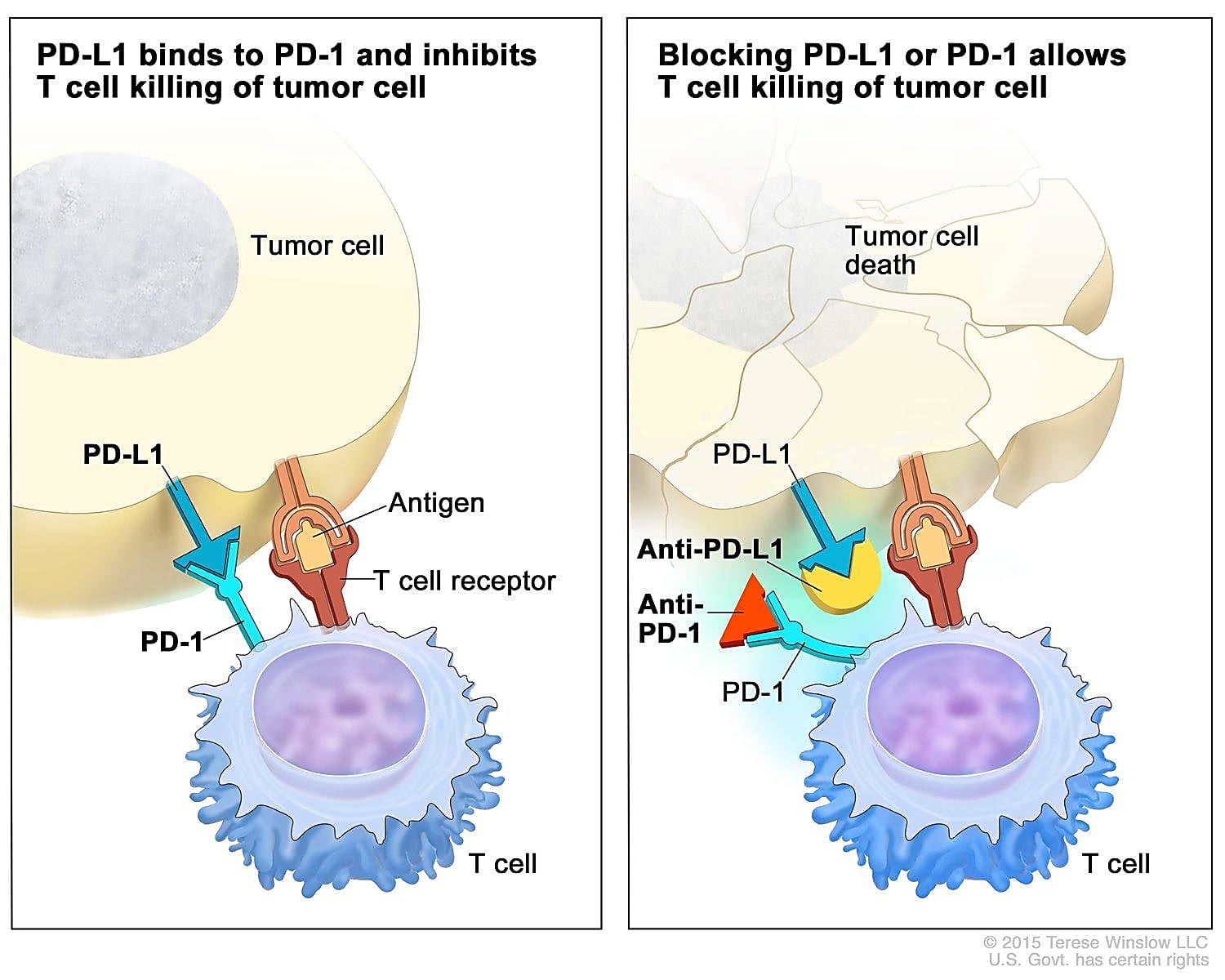
A mere 15 years ago, less than 20% of patients lived five years post–lung cancer diagnosis; those with later stage disease typically only lived for a few more months (2). While treating lung cancer has changed remarkably for the better with the introduction of ICIs—the first, nivolumab, was approved for treating lung cancer in 2014 (3)—most patients don’t show a long-lasting clinical response (4). Therefore, improving our understanding of T-cell dysfunction as a tumor progresses and in response to treatment is critical to improving the efficacy and duration of this type of therapy.
In recent work (5), scientists led by Jason M. Schenkel, PhD, MD, at Harvard Medical School used a blend of traditional research tools and single cell RNA sequencing (scRNA-seq) to discover that the tumor-draining lymph node acts as a reservoir of one subpopulation of T cells—allowing his team to make the case for targeting the T-cell trafficking pathway to boost response to checkpoint blockade therapy.
Single cell transcriptomics helps define and characterize the TCF-1+ CD8+ T-cell population
Effector CD8+ T cells are the main cancer-killing cells acting on tumors. However, as tumors persist, CD8+ T cells become increasingly dysfunctional, begin to express inhibitory receptors, and stop being able to proliferate, secrete cytokines, and kill tumor cells (6). Studies have discovered two subsets of dysfunctional CD8+ T cells, one of which expresses the transcription factor TCF-1 and is the preferred target of checkpoint blockade (7). In this study, Dr. Schenkel’s team used a genetically engineered mouse model of lung adenocarcinoma to monitor TCF-1+ CD8+ T cells as the tumor progressed over the course of months.
Using a combination of traditional and single cell tools, they found that CD8+ T cells become more dysfunctional as the tumor progresses. Employing Chromium Single Cell 5’ Gene Expression to perform transcriptomic profiling on CD8+ T cells from cancerous lung tissue at 6, 7, 8, and 12 weeks after initiating tumors to grow, they observed that key gene expression programs changed over time. One of these programs was a “canonical dysfunctional-like program” and another was a “quiescent-like program,” which includes Tcf7. Not only were the cells within this T-cell population heterogeneous, but their gene expression patterns increasingly appeared more quiescent (versus effector-like) as the tumor progressed.
Their single cell transcriptomic analysis also uncovered two functionally distinct subpopulations of SlamF6, an immunoglobulin that acts to regulate the immune response, within the TCF-1+ CD8+ T-cell population. They found a proliferative SlamF6+ population and a non-cycling SlamF6– population, and they believe these two types of cells may play different roles within the tumor microenvironment.
Targeting a peripheral reservoir of cells to improve therapeutic response to checkpoint blockade
They followed up their single cell analysis with several functional assays to discover that the SlamF6+ T-cell population exists in the tumor-draining lymph node (dLN), which acts as a reservoir of these cells. By functionally increasing conventional type I dendritic (cDC1) cells, which are crucial regulators of the CD8+ T-cell response, they could increase stimulation of the SlamF6+ cells in the dLN. This in turn led to better T-cell trafficking into the tumor and improved immune response.
By employing single cell analyses, the team was able to show that there is a functional subset of tumor-killing cells stored within the dLN, and that cDC1 cells could possibly be targeted to harness the anti-tumor response of T cells to checkpoint blockade therapy. While they are not the first researchers to determine how important the peripheral immune response is in the response to immunotherapy (8), they hope their work will further help characterize critical subpopulations of cells that may be key to improving the efficacy and durability of checkpoint blockade therapy for treating lung cancer.
References:
- https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html
- https://www.nature.com/articles/d41586-020-03154-y
- Keisuke O, et al. Immune checkpoint inhibitors for lung cancer treatment: A review. J Clin Med 9: 1362 (2020). doi: 10.3390/jcm9051362
- Ribas A & Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 359: 1350–1355 (2018). doi: 10.1126/science.aar4060
- Schenkel JM, et al. Conventional type I dendritic cells maintain a reservoir of proliferative tumor-antigen specific TCF-1+ CD8+ T cells in tumor-draining lymph nodes. Immunity 54: 2338–2353.e6 (2021). doi: 10.1016/j.immuni.2021.08.026
- Blank CU, et al. Defining ‘T cell exhaustion’. Nat Rev Immunol 19, 665–674 (2019). doi: 10.1038/s41577-019-0221-9
- Kurtulus S, et al. Checkpoint blockade immunotherapy induces dynamic changes in PD-1– CD8+ tumor-infiltrating T cells. Immunity 50: 181–194.e6 (2019). doi: 10.1016/j.immuni.2018.11.014
- Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). doi: 10.1038/nature22079
