Making memories: Microglia clear the way for long-term fear memory consolidation
Scientists from the University of California, San Francisco are shedding light on the molecular mechanisms involved in fear-memory formation, one of our more mysterious cognitive functions. They identified IL-33 as a signaling molecule from hippocampal neurons that triggers a microglia-mediated cleanup process of the synaptic spaces between neurons, an essential function to ensure synaptic plasticity and consolidation of fear memories. Read this blog to learn how single cell sequencing played a part in revealing these new insights into what makes a memory.
Imagine if our minds didn’t remind us what to be afraid of. We tend to learn through experiences—touching a hot surface, a near accident in a car—but if we couldn’t consolidate the lessons from these moments and build associations that guide our future behavior, we’d run the risk of responding to our environments inappropriately or in a way that would put us in danger.
Fear, though an unpleasant emotion, is incredibly important for learning through memory consolidation. Classical conditioning experiments in animal models show us as much. Izquierdo et al. said, “Fear conditioning is a fundamental form of learning through which animals learn to predict aversive events and to react appropriately to threats” (1). The human mind employs the same learning tactics:
“The capability of our brains to form a fear memory associated with a situation that predicts danger is highly adaptive since it enables us to learn from our past traumatic experiences and avoid those dangerous situations in the future” (2).
On the other hand, when the process of fear-memory formation is dysregulated, you can experience “overgeneralized and exaggerated fear responses...including nightmares or unwanted memories of the trauma, avoidance of situations that trigger memories of the trauma, heightened reactions, anxiety, and depressed mood” (2), in the case of post-traumatic stress disorder (PTSD). Thus, fear-memory formation, a double-edged sword, is more than nerves. It’s a complex neural process with psychological and physiological impact that is integral to development and survival, but also vulnerable to dysregulation as a result of our experiences or age-related decline in memory precision.
How fear memories are created: Exploring the role of IL-33
Neuroscientists have tracked down the memory-making machinery in our brains. Central to that function is the hippocampus. Here, the connections between neurons, or neuronal synapses, change in response to experience, a regular structural reorganization process that is essential for learning and memory consolidation. Growing evidence suggests immune cells in the brain, namely microglia, interact with neurons to drive this synaptic reorganization (3). However, it is still unclear exactly how microglia and neurons collaborate and what molecules regulate their interactions to remodel synapses and enable our minds to encode fear memories.
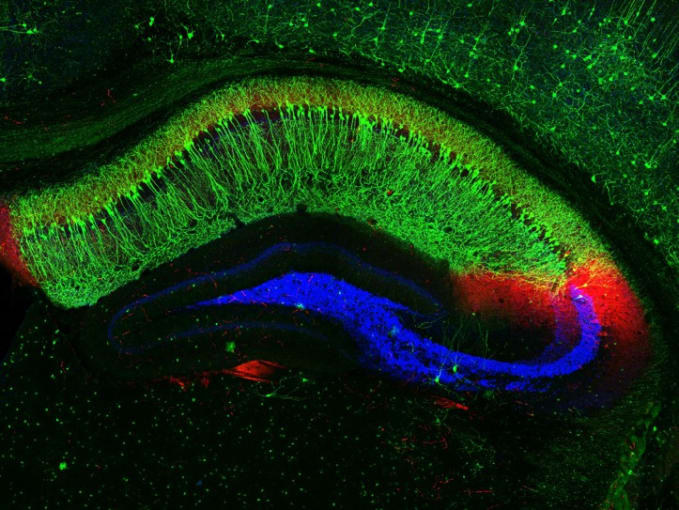
In an effort to define the underlying mechanisms of experience-driven memory formation, Dr. Phi Nguyen and colleagues from the University of California, San Francisco (UCSF) assessed the role of cytokine interleukin-33 (IL-33), previously shown to drive microglial synapse engulfment in the thalamus and spinal cord (3), in controlling microglia activity. Now, the research team is investigating how experiences may regulate Il33 expression, and in turn, control microglial interactions with neuronal synapses.
Using knock-in mice expressing nucleus-localized, fluorescently tagged Il33, the research team took sections of the hippocampus and observed that a majority of the cells expressing Il33 were neurons. Specifically, Il33 was expressed by approximately 74% of neurons in the dentate gyrus (DG) and 24% of neurons in the CA1 subregions of the hippocampus.
They next assessed how Il33 expression changed in young mice who had an enriched environment, which encourages neural circuit remodeling and neurogenesis, versus those in an environment marked by social isolation. Could experience dictate Il33 expression patterns, and so reveal a link between Il33 and synaptic remodeling? Their study revealed that after 4 weeks, mice from the enriched environment had a significant increase in the proportion of Il33+ CA1 neurons, while socially isolated mice had significantly reduced overall Il33 mRNA expression and a lower percentage of Il33+ neurons in the DG (3). This pointed to a role for Il33 expression, or the absence thereof, as a result of positive and negative experiences in controlling synaptic plasticity.
Single cell RNA-seq identifies the neuronal source of IL-33 in the hippocampus
Studies of cellular heterogeneity show us that every cell is unique, and the team noted that neuronal populations in the DG expressed varying levels of IL-33. To unmask neuronal heterogeneity in the hippocampus, and build a more complete picture of cell type–specific Il33 expression patterns, the team leveraged single nucleus RNA-sequencing. This revealed two distinct neuronal populations corresponding to DG granule cells and several other major classes of neurons. Single cell sequencing of a flow-sorted population of Il33+ neurons from the hippocampus revealed that the majority of Il33+ neurons overlapped with the second DG cluster. Differential gene expression analysis showed that the Il33-enriched cluster expressed genes linked to cell adhesion, synapse assembly, and synaptic plasticity, as well as 18 genes related to assembly and remodeling of the extracellular matrix (ECM), including Adamts9, Itga4, Col23a1, and Col25a1. This revealed a possible role for the DG subtype in coordinating IL-33-mediated synapse formation and ECM remodeling.
Neurons and microglia: Working together to form memories
Neural signaling requires an input and a receiver: what cells receive IL-33 to mediate synaptic remodeling and its downstream effects, memory formation? To understand this, the research team looked for cellular targets of IL-33 signaling, tracking the cell types that expressed Il1rl1, which encodes the IL-33 co-receptor. Flow cytometry and in situ hybridization data pointed them to microglia. They further showed that loss of either neuronal IL-33 or microglial IL1RL1 resulted in fewer dendritic spines with spine head filopodia, projections that branch off from neurons and help transmit electrical signals to the neuron cell body. In contrast, enrichment of IL-33 and IL1RL1 increased the number of spine head filopodia, suggesting that neuron-microglia signaling via IL-33 promotes spine formation and plasticity, which is, in turn, crucial for neuronal signaling.
Finally, what do microglia really do to promote synaptic plasticity? To answer this question, the research team investigated the impact of IL-33 signaling on hippocampal microglial transcription. After 4 hours of in vivo exposure to IL-33, they noted several prominently upregulated genes, including the class A scavenger receptor Marco, a regulator of filopodial morphogenesis and debris clearance, and Gas7, which encodes an adaptor protein necessary for phagocytosis (3). Additionally, they found that 76 genes differentially expressed in microglia after IL-33 treatment were associated with the ECM, including several ECM proteases like Adamts4, Mmp14, Mmp25, and Ctsc. This indicated that microglia likely mediate synaptic plasticity via IL-33 induced phagocytosis and clearance of ECM proteins.
Tracing the path of fear-memory consolidation
From experience to synapse reorganization—and the crucial molecular signaling and cellular interactions in between—the path to fear-memory formation is becoming increasingly clear with the help of innovative research and tools. Thanks to single cell sequencing, new insights into the various roles of IL-33 signaling, neuron–microglia interactions, and ECM protein clearance in enabling synaptic plasticity not only reveal mechanisms underlying memory-formation, but provide important opportunities to understand how these processes can go amiss in age-related decline and neuropsychiatric disorders.
References:
- Izquierdo I, et al. Fear memory. Physiol Rev 96: 695–750, 2016.
- University of California - Riverside. How associative fear memory is formed in the brain: Insights into how pathological fear memory in PTSD could be suppressed. ScienceDaily, 2020. www.sciencedaily.com/releases/2020/03/200313112137.htm.
- Nguyen PT, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182: 1–16, 2020.
