Recent Study Published in Nature Unravels a Novel Pathway for Stem Cell Self-Renewal
The renewal and differentiation of Lgr5+ intestinal stem cells (ISCs) is critical to the continuous regeneration of the epithelial lining of the gut, which enables us to absorb nutrients and provides a barrier to protect us from the external environment. Disruptions in this process can lead to or worsen human intestinal disorders such as inflammatory bowel disease (IBD), gastrointestinal cancer and Celiac disease. In a recent Nature publication, "Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal", Yan et al., investigate the roles of Wnt and R-spondin (RSPO1-RSPO4) ligands in ISC regeneration of the epithelium to better understand ISC proliferation and differentiation. Performing gain of function (GOF) and loss of function (LOF) in vivo studies along with single-cell RNA-seq, the researchers were able to tease out the roles of Wnt and RSPO ligands and develop a novel model for ISC proliferation and differentiation.
The intestinal epithelium as a model for tissue regeneration
The intestinal epithelium is the most rapidly renewing tissue in mammals, making it an ideal model for tissue regeneration. Composed of a single layer of epithelial cells and organized into villi (finger-like projections) and crypts, the intestinal epithelium has rapid cellular turnover every 3-5 days. The intestinal crypt compartment is made up of stem cells that not only self-renew but also give rise to lineage-committed progenitor cells that undergo proliferation and rise up the crypts to the villi where they undergo terminal differentiation into 1 of 4 major lineages of the intestine (see diagram below). The differentiated cells continue the upward migration until approximately 4 days later when they are sloughed off into the lumen.
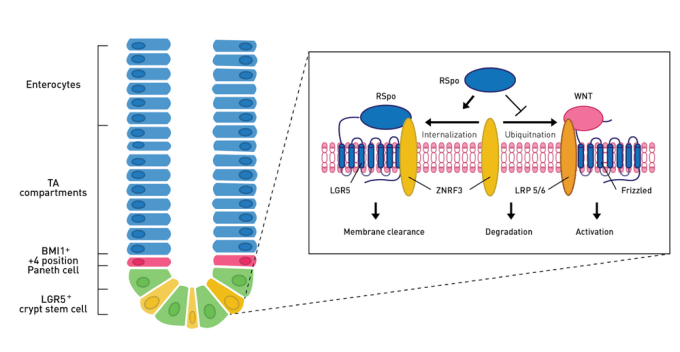
The Wnt/**ß-catenin signaling pathway governs ISC proliferation and differentiation**
The canonical Wnt/ß-catenin signaling pathway has been well studied and is known to be important for the maintenance of intestinal epithelial turnover, as Wnt inhibition results in loss of intestinal crypts and destruction of the intestinal epithelium. Newly described ligands within the Wnt pathway, R-spondins are able to potentiate ß-catenin signaling, and in vivo overexpression studies indicate RSPO drives Lrg5+ ISCs to expand and proliferate.
Despite what is known about Wnt and RSPO, further dissecting the roles of each ligand had proven difficult:
- Wnts and RSPOs are structurally distinct classes of extracellular ligands that coordinately regulate the same pathway
- Both Wnt and RSPO are redundant
- 19 Wnts bind to 10 different cell-surface frizzled (FZD) receptors, in addition to LRP5 and LRP6 co-receptors
- 4 RSPOs bind to distinct LGR4-LGR6, RNF43 and ZNRF3 receptor classes
- GOF studies in vivo with Wnt have been limited by difficulties in protein production and poor bioavailability
Creating a toolbox to dissect the role of Wnt v. RSPO ligands
To help deconstruct and dissect the effects of Wnt v. RSPO ligands, Yan et al. built a toolbox of pharmacological reagents for Wnt pathway modulation. For LOF studies, the team utilized soluble receptors that were expressed via modified adenovirus infection of the mice to bind and sequester Wnt or RSPO ligands. To overcome the challenges associated with Wnt GOF studies, the researchers collaborated with another lab to engineer a diffusible, non-lipidated, artificial Wnt ligand, referred to as a Wnt analogue. This novel Wnt analogue was tested in vitro and in vivo and confirmed to be soluble and freely diffusible in peripheral blood. Armed with their toolbox, Yan et al. set out to elucidate the roles of Wnt and RSPO ligands in ISC proliferation and differentiation.
Wnt and RSPO ligands are not functionally equivalent
RSPO ligand LOF experiments showed loss of the Lgr5+ cells in the crypts when either the soluble LGR ECD receptors or RNF43 and ZNRF3 co-receptors were expressed; however, simultaneous overexpression of RSPO ligands was able to reverse the effects. Further fate mapping of the Lgr5+ ISCs upon RSPO ligand inhibition showed precocious lineage commitment with disappearance of the Lgr5+ ISCs by day 4. Overexpression of RSPO ligands resulted in a massive enlargement of the crypt by day 4. In this case, lineage commitment was blocked and the Lgr5+ ISCs were driven into a state of self-renewal only. The results of the GOF and LOF studies led researchers to the idea that RSPO actively controls the cell fate decision of Lgr5+ ISCs between self-renewal and lineage commitment.
To investigate whether Wnt ligands act similarly to RSPO ligands, GOF and LOF studies were performed in vivo. LOF studies resulted in rapid lethality within 5-7 days, showing complete loss of the stem cell and crypt compartments. GOF studies with the novel Wnt analogue showed no definable phenotype in the intestine. However, Wnt analogue and RSPO1 overexpression together resulted in a massive increase in proliferation, but no expansion in the ISC compartment in comparison to what was observed with RSPO overexpression alone. These results indicated that RSPO ligands induce ISCs (not Wnt) and also specify the size of the stem cell pool.
Further in vivo experiments demonstrated that Wnt and RSPO ligands are not interchangeable. When RSPO ligand activity is inhibited in the presence of Wnt overexpression, the crypts are rescued, but not the stem cells. And, when Wnt ligand activity is inhibited in the presence of RSPO overexpression, the loss of crypts cannot be rescued. Thus, Wnt and RSPO ligands are not functionally equivalent, with Wnt conferring a priming or competency step for self-renewal, while RSPO actively induces self-renewal and specifies the size of the ISC pool.
Using single-cell analysis to confirm observed phenotypes
Yan et al. used 10x single-cell analysis to examine what was happening to the ISC populations on a molecular level under the LOF and GOF conditions. Using the long half-life in the GFP-labeled Lgr5+ ISCs, the researchers were able to track and isolate the cells for analysis. The team looked at the gene expression in non-stem cell and stem cell controls, as well as, 5 different GOF/LOF conditions of ISCs. For each condition, they analyzed ~1800 cells and detected ~2500 genes/cell. Consistent with phenotypic observations, Wnt analog overexpression showed no perturbations in the make-up of the ISC population compared to normal controls. LOF of Wnt or RSPO ligands showed a loss of the stem cell cluster and an increase in transient amplifying cells, which is consistent with precocious lineage differentiation. RSPO ligand GOF conditions showed stabilization of stem cell states and a depletion of transient amplifying cells. The Wnt LOF condition also showed a decrease in RSPO receptors (among other transcripts).
Developing a novel model for ISC proliferation and differentiation
Yan et al. define a new model for ISC proliferation and differentiation in which active signals are required to maintain the ISCs in a state of self-renewal, as differentiation is the default state. The data show that Wnt primes cells by maintaining RSPO receptor expression, and RSPO ligands act upon Wnt-primed Lgr5+ ISCs to induce self-renewal and expand the ISC pool. This new model has broader implications for how other mammalian stem cell compartments may be regulated.
Additional Resources
- Read the press release
- Learn more about the Chromium™ Single Cell 3' Solution
- Read single cell application notes
- Check out our data analysis and visualization tools - Cell Ranger and Loupe Cell Browser
- Download single cell datasets
