scRNA-seq suggests a new combination therapy recruits stem cell populations favorable for autologous stem cell transplants in cancer patients
Impact at a glance: A new combination therapy (motixafortide with filgrastim) helps mobilize better stem cell populations for transplants in multiple myeloma patients, making it easier to collect high-quality cells and offering hope for improved outcomes. Single cell sequencing reveals why this therapy works and how it might help guide future treatment advances for blood cancers.
Successful clinical trials take many forms. Some identify transformative therapeutics, while others fail to meet primary endpoints but yield key biological insights to guide future drug discovery efforts. In our Clinical trials to watch blog series, we feature innovative applications of single cell multiomics and spatial gene expression to clinical samples across trial phases.
In this blog, we discuss how researchers identified populations of hematopoietic stem and progenitor cells (HSPCs) that drive successful autologous stem cell transplantations (ASCT) through single cell RNA sequencing (scRNA-seq) analysis of patient samples from an international, multicenter phase 3 clinical trial that tested the improved efficacy of a new combination therapy for HSPC mobilization in people with multiple myeloma (MM).
The results of the trial, published in Nature this April, demonstrated that BioLineRx’s lead investigational candidate motixafortide combined with filgrastim (granulocyte colony-stimulating factor (G-CSF)) to mobilize HSPCs for ASCT—the standard drug for HSPC mobilization—was more effective at mobilizing stem cells for ASCT in patients with MM (1).
The FDA approved this combination therapy in September. In a press release following the approval, BioLineRx stated APHEXDA (motixafortide) is “the first innovation in stem cell mobilization for MM to be approved in the U.S. in a decade” (2).
But the innovation didn’t end with an FDA-approved treatment. scRNA-seq analysis of patient samples collected during this trial provided some of the first biological insights into what populations of HSPCs are most favorable for long-term ASCT success.
Phase 3: A peptide inhibitor improves HSPC mobilization to the blood
Cancer type: Multiple myeloma
Therapy: APHEXDA (motixafortide) in combination with filgrastim (G-CSF)
Trial aim: To evaluate the safety and efficacy of combining APHEXDA with filgrastim, compared to placebo plus filgrastim, to mobilize HSPCs for ASCT in patients with multiple myeloma
Leading institutions: Washington University School of Medicine in St. Louis & UCLA School of Medicine
Pharmaceutical collaborators: BioLineRx
ClinicalTrials.gov registration: NCT03246529
Why is HSPC mobilization critical for effective ASCT in patients with multiple myeloma?
HSPCs maintain and repopulate blood during development and through adulthood. Chemotherapy kills these rapidly dividing, self-renewing cells along with their derivatives, including leukocytes, red blood cells, and platelets (3).
HSPC transplants significantly improve overall survival in people with blood cancers like multiple myeloma after chemotherapy or radiation. Clinicians can collect grafts from bone marrow or peripheral blood from either the transplant recipient or a matched donor. Although HSPCs primarily reside in the bone marrow during adulthood, blood grafts are more common (3, 4).
Bone marrow collection can be painful and invasive, often requiring an operation under anesthesia. However, collecting blood is nearly painless which makes the process more amenable for unrelated donors, and some studies suggest engraftment is faster and more likely to succeed with peripheral blood grafts collected from either a donor or the patient (5).
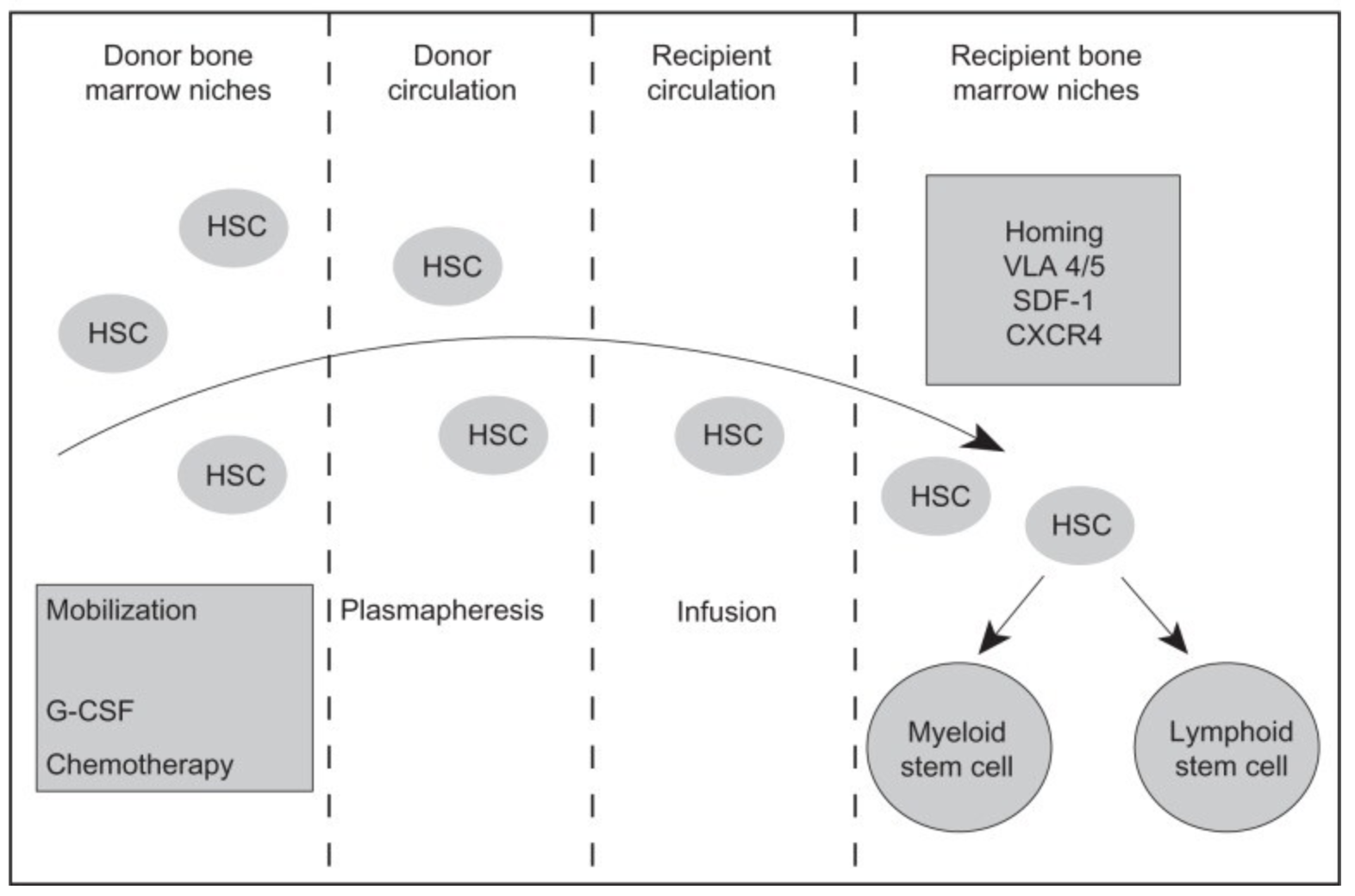
No matter the graft source, efficacy is ultimately dependent on the number and quality of cells that can be collected, and current stem cell mobilization strategies are often unable to mobilize enough cells. For example, a sufficient number of HSPCs are collected from only 40–50% of people with MM following G-CSF treatment, the current standard treatment for HSPC mobilization (6).
How does the mechanism of action of motixafortide promote HSPC mobilization?
Scientists collect HSPCs from the blood from people with MM via apheresis—a process by which plasma and cells are separated. HSPCs mobilize to peripheral blood when the cell surface receptor CXCR4 is unoccupied; when CXCL2 is bound to the receptor, HSPCs stay in the bone marrow (7). Motixafortide is a peptide inhibitor that binds CXCR4 and inhibits CXCL2 binding, which promotes HSPC mobilization to peripheral blood. Motixafortide is a particularly promising CXCR4 inhibitor due to its high affinity for the CXCR4 receptor and long-lasting effects (1). For example, CXCR4 inhibitor plerixafor also improves HSPC mobilization, but it has low affinity to the CXCR4 receptor and is short acting (8).
Does improved HSPC mobilization improve ASCT outcomes?
Not necessarily. If clinicians can mobilize more HSPCs more quickly in a greater number of patients, it makes ASCT more accessible, but not necessarily more effective. The types of HSPCs mobilized seem to matter as much, if not more, than the number of transplanted cells.
Researchers previously assumed cell surface protein CD34+ was a sufficient immunophenotypic biomarker for sorting HSPCs from peripheral blood. However, studies using flow cytometry and scRNA-seq to analyze clinical samples suggested CD34+ HSPCs are highly heterogeneous, and some cell subsets are likely more favorable for ASCT than others, particularly primitive HSPCs—stem cells able to self-renew long-term while maintaining the ability to differentiate to a variety of hematopoietic cells (9).
Previous studies demonstrated plerixafor mobilized a variety of CD34+ HSPC subsets, including primitive and multipotent HSPCs, but a deep characterization of these subtypes and their effects on the long-term outcomes of people who received ASCTs was not done. A more detailed understanding of whether or not CXCR4 inhibitors, like motixafortide, mobilize the more primitive HSPCs is an open question. This clinical study testing combined treatment of motixafortide and G-CSF is one of the first to deeply interrogate the cell populations this class of drugs mobilizes.
Clinical results
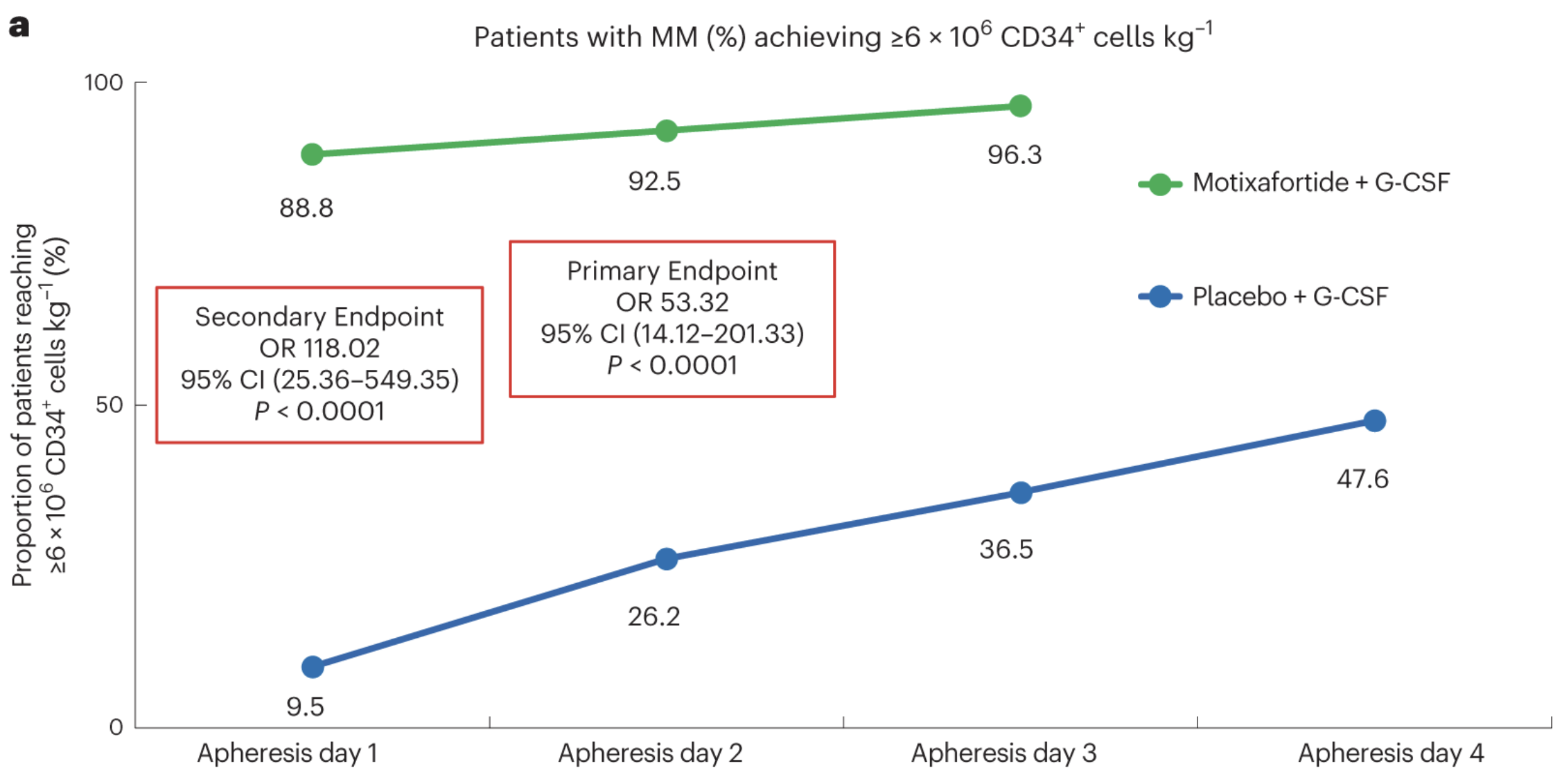
122 people with MM across 18 sites were randomized and treated with either motixafortide + G-CSF (80 participants) or placebo + G-CSF (42 participants). Scientists collected the optimal number of stem cells for ASCT (≥6 x 106 CD34+) from 92.5% participants treated with motixafortide after the second day of apheresis, meeting the primary endpoint. 88% only required one day of apheresis after treatment, meeting the secondary endpoint. Only 47% of those treated with placebo reached the primary endpoint after 4 days of apheresis—no patients treated with motixafortide required a fourth round of apheresis. Graft durability, progression-free survival, and overall survival were not significantly different 100 days after ASCT between participants transplanted with HPSCs collected following treatment with G-CSF alone or motixafortide + G-CSF.
Although survival did not seem to improve following transplantation of HSPCs collected from participants treated with the experimental combination therapy compared to conventional G-CSF treatment, the researchers believe this new mobilization therapy will improve survival in people with MM in the long run based on the results of single cell analysis of samples collected from clinical trial participants.
Applications of single cell sequencing
The authors used Chromium Single Cell 5’ Gene Expression to identify the variety and prevalence of CD34+ HSPC subsets—particularly primitive HSCs—in clinical samples from 12 people with MM treated with motixafortide + G-CSF, plerixafor + G-CSF, or placebo + G-CSF to understand the mechanism driving improved HSPC mobilization observed with motixafortide + G-CSF in the clinical trial.
They also analyzed clinical samples from 6 healthy donors without MM treated with motixafortide, plerixafor, or G-CSF alone to further elucidate the cell populations mobilized by motixafortide. As of 2019, ~80% of all hematopoietic stem cell transplants use samples collected from unrelated donors so examining the efficacy of motixafortide in healthy individuals is imperative.
Using uniform manifold approximation and projection (UMAP) clustering, the researchers identified 20 transcriptionally distinct CD34+ HSPC subsets based on known lineage markers. The authors focused most of their downstream analyses on the 6 cell subsets with transcriptional profiles akin to haematopoietic stem cells.
The researchers then determined what stage each cell subset was in during differentiation from a haematopoietic stem cell to a mature lineage cell (e.g., lymphocytes and erythrocytes). scRNA-seq analysis uniquely enables the identification of cells in transient states when genes are rapidly and dynamically regulated to move the cell forward to the next stable cell state (e.g., hematopoietic stem cell to myeloid progenitors).
Cells in these transient states are difficult to isolate by classical methods like flow cytometry, which can only sort cells based on proteins, primarily cell surface proteins. Immunologists historically used cell surface proteins, like CD34+, to categorize immunological subsets. But cell surface markers do not change in a significant way between one cell type to another—like the transition from an HSC to an HPC—leaving these transient states undetectable. Single cell sequencing, however, enables researchers to analyze transcript levels from individual cells in samples with actively differentiating cells, giving researchers the resolution needed to parse transient cell states.
For example, a classic diagram of hematopoietic stem cell differentiation often presents various differentiation trajectories, including stem cell differentiation to a lymphoid progenitor, which differentiates into natural killer cells and lymphocytes (see Figure 1). But the arrows pointing from one illustrated cell to another fail to capture the countless, small transcriptional adjustments the cell made to get there.
The researchers employed the software analysis tool Monocle 3 to perform trajectory analysis over pseudotime, which predicts where a cell is in the differentiation process at a given point in time (10). Briefly, Monocle 3 uses an algorithm to learn the sequence of gene expression changes that occur as primitive stem cells differentiate into lineage-committed cells—to perform trajectory analysis over pseudotime. Once the algorithm learned the overall "trajectory" of gene expression changes, it determined the position of each individual cell in the trajectory.
This analysis predicted the cell subset HSC1 was the most transcriptionally primitive cell, differentiating into HSC2-6 and myeloid progenitor cells (MLP). The remaining subsets originated from HSC6 or MLP.
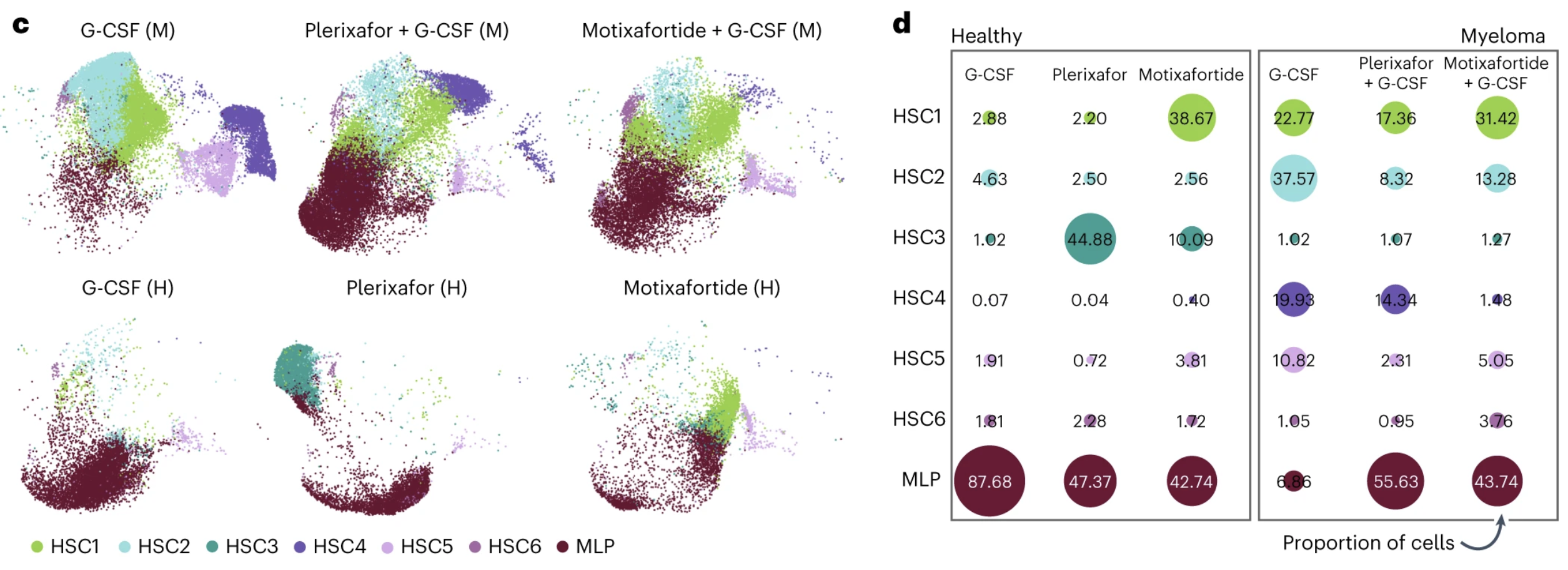
The small adjustments the cells made across these seven cell states added up to big differences in the cell makeup between cohorts and treatments. The proportion of primitive HSC1 cells in samples from healthy donors treated with only motixafortide was more than 25% compared to less than 2% in those treated solely with plerixafor or G-CSF. The proportion of HSC1 cells was also higher in people with MM treated with motixafortide + G-CSF (31.42%) than those treated with plerixafor + G-CSF (17.36%) or placebo + G-CSF (22.77%).
Differential gene expression analysis comparing CD34+ HSPC subsets between mobilization regimens and donor types confirmed the HSC1 population was enriched in those treated with motixafortide compared to plerixafor or G-CSF.
Specifically, they described increased expression of genes previously associated with quiescence, HSC maintenance, and self-renewal in those treated with motixafortide. Gene set enrichment analysis corroborated these findings, revealing increased signaling in key pathways involved in hematopoietic regeneration and regenerative potential.
Overall, the scRNA-seq analysis indicated that motixafortide mobilized a favorable composition of cell subsets that may be associated with long-lived HSPCs that could improve ASCT efficacy. Although the results of the clinical study did not identify significant differences in graft durability or patient survival between transplants of motixafortide + G-CSF or G-CSF only mobilized HSPCs 100 days after ASCT, the authors pointed out that these results bring them hope that their breakthrough mobilization therapy will translate into improved ASCT efficacy overall.
"The cohort analyses were not designed to understand potential clinical outcomes; nevertheless, we believe the findings are of interest and a compelling area for further research," said John DiPersio, MD, PhD, Professor of Medicine and Director of the Center for Gene and Cellular Immunotherapy at Washington University School of Medicine in St. Louis and principal investigator of the GENESIS study.
Read the full clinical trial publication
Learn more about the technology that supported these clinical findings—Chromium Single Cell Gene Expression
References:
- Crees ZD, et al. Motixafortide and G-CSF to mobilize hematopoietic stem cells for autologous transplantation in multiple myeloma: a randomized phase 3 trial. Nat Med 29: 869–879 (2023). DOI: 10.1038/s41591-023-02273-z
- https://www.prnewswire.com/news-releases/biolinerx-announces-fda-approval-of-aphexda-motixafortide-in-combination-with-filgrastim-g-csf-to-mobilize-hematopoietic-stem-cells-for-collection-and-subsequent-autologous-transplantation-in-patients-with-multiple-myeloma-301923206.html
- https://www.cancer.gov/about-cancer/treatment/types/stem-cell-transplant#:~:text=in%20clinical%20trials-,Types%20of%20cancer%20treated%20with%20stem%20cell%20transplants,used%20to%20treat%20certain%20cancers
- Hatzimichael E & Tuthill M. Hematopoietic stem cell transplantation. Stem Cells Cloning 3: 105–117 (2022). DOI: 10.2147/SCCAA.S6815
- https://ashpublications.org/ashclinicalnews/news/5761/Marrow-or-Peripheral-Blood-for-Allogeneic
- Demirer T, et al. Factors influencing collection of peripheral blood stem cells in patients with multiple myeloma. Bone Marrow Transplant 17: 937–941 (1996).
- Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 201: 1307-18 (2005). DOI: 10.1084/jem.20041385.
- DiPersio JF, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 113: 5720–6 (2009). DOI: 10.1182/blood-2008-08-174946.
- Schroeder MA, et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood 129: 2680–2692 (2017). DOI: 10.1182/blood-2016-09-739722
- https://cole-trapnell-lab.github.io/monocle3/docs/trajectories/
