Time plus technology reveal secrets of long-lasting CAR T therapy
In 2010, two patients received CAR T-cell therapy for advanced chronic lymphocytic leukemia, experiencing total remission in mere months. In 2021, both were still cancer free. The secret to long-term treatment success has been a mystery, until now. Using single cell immune profiling technology from 10x Genomics, the authors behind the original clinical study have investigated how the CAR T cells have evolved over the past decade to provide continuous protection from the return of cancer—amazing results that represent a potential step towards finding a cure.
Drug mechanisms: When understanding trails treatment
“Here’s how we actually develop a surprising number of treatments: good old-fashioned observation, trial and error, and luck. Detailed scientific understanding of how a drug works often comes, ironically enough, near the end of the process” (1).
As this quote demonstrates, despite popular perception, scientists and doctors don’t always know exactly how drugs work to alleviate the complex symptoms of the diseases they treat. Deep understanding of drug mechanisms of action can, surprisingly often, follow treatment success.
“Knowing why a drug works has historically trailed the treatment, sometimes by decades. Some of the most recognizable drugs—acetaminophen for pain relief, penicillin for infections, and lithium for bipolar disorder, continue to be scientific mysteries today” (1).
Why might this be the case? For one, present needs of patients can drive innovative decisions in real time. As Pasteur was quoted, “Fortune favors the prepared mind” (1). More robust trials and, with that, time, are generally needed to build up mechanistic understanding too. Available technology can also be a limiting factor. Research tools at the time of implementation may not be advanced enough, lacking the needed resolution to understand the cellular and molecular basis of drug efficacy.
Evolution of CAR T, a living therapy
A recent example of this surprising feature of the drug development process comes out of a study of the long-term efficacy of the first ever CAR T-cell infusions (2), a cancer immunotherapy approach that helped two men in a 2010 clinical trial experience over 10 years of remission from their leukemia.
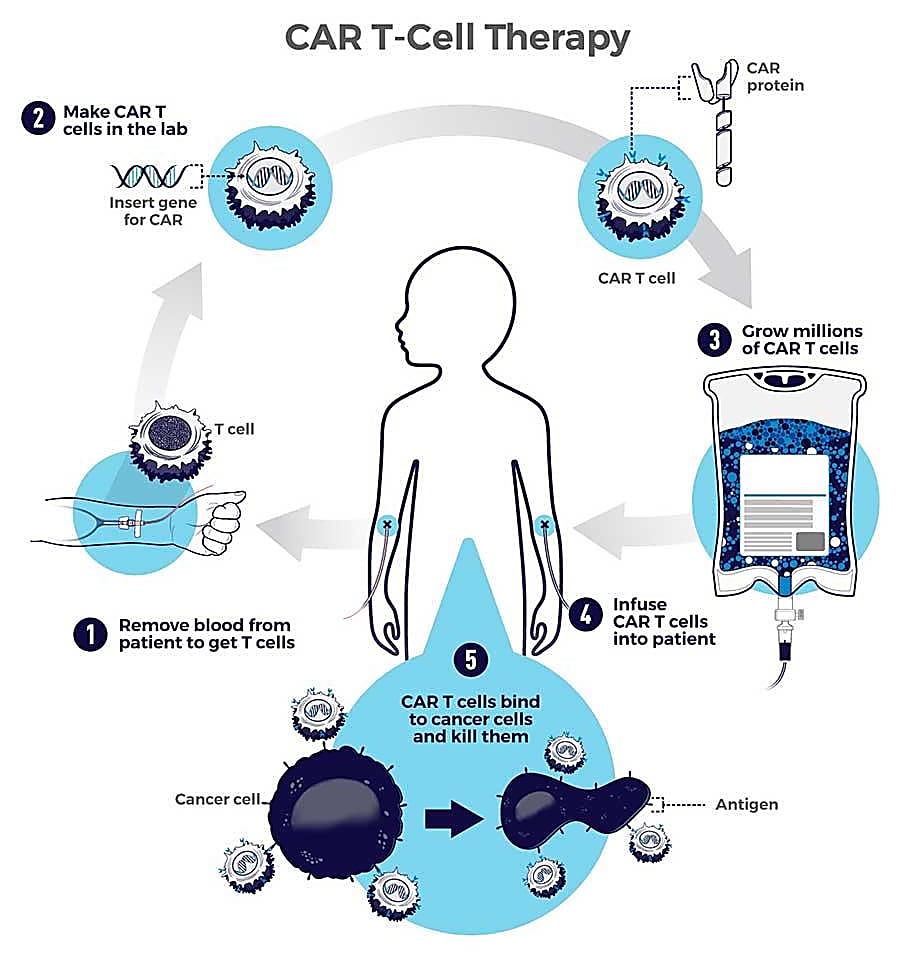
CAR T cells are T cells taken from the patient’s own body and engineered to express a synthetic receptor known as a CAR, or chimeric antigen receptor, enabling a targeted attack against cancer cells once the T cells are reintroduced into the body (3, 4).
Initial observations of the treatment in 2010 showed potent antileukemic effects in the patients, with quantitative PCR assays detecting expansion of CAR T cells and persistence in the blood and bone marrow for at least 6 months after treatment (5). Multicolor flow cytometry was used to functionally characterize the CAR T cells, revealing unique compartments of CD4+ cells that appeared to acquire features of a central memory phenotype—T cells in a naive-like state, yet sensitive to cancer antigen stimulation (6)—and CD8+ cells with a strong effector memory profile, meaning they had experienced prolonged and robust exposure to antigen. These early observations were hopeful, suggesting a mixed population of central and effector memory T cells could support CAR T-cell persistence (5). However, the question remained—for how long? Could the CAR T cells persist, even in the eventual absence of cancer antigen? If they did, why?
Jump forward to 2021.
“Ten years on, no leukemia cells, and we still have CAR-T cells that are on patrol and surveillance from leukemia” (Dr. Carl June; 3).
Many other cancer patients have been treated with CAR T therapy since 2010, yet few have experienced these lasting results. More often, patients experience a relapse of their cancers, making an investigation into the underlying biology of successful cases all the more urgent (3). This led a team of scientists from the University of Pennsylvania Perelman School of Medicine, including Dr. Carl June, Director of the Center for Cellular Immunotherapies, and lead author Dr. J. Joseph Melenhorst, Research Professor at Perelman, to take a fresh approach to some old samples—cryopreserved blood samples from the two patients who experienced complete remission.
Looking to assess the long-term potential and clonal stability of the infused CAR T cells, they first isolated CAR+ cells from 10 longitudinal samples, taken consecutively from both patients and ranging from 1 month to over 9 years after treatment (2). Then, they used a combination of technologies, including single cell RNA-sequencing with cell surface protein profiling and immune repertoire sequencing, to capture a comprehensive profile of the CAR T cells. Single cell analysis revealed some surprising and previously undetected features of the CAR product that may play a major part in the long-term success of the cellular therapy.
CAR T details revealed by single cell immune profiling
Surprise cytotoxic CD4+ CAR T cells dominate late-stage response
Initially using CyTOF data, the team was able to identify a clear shift in CAR T-cell composition in longitudinal samples over time, marked by a CD8+ to CD4+ transition. For example, in one patient they saw that 15.9% of CAR T cells were CD4+ at the 1.8 month time point, increasing to 97% by year 9 post-treatment (2). CD8+ CAR T cells exhibited early stage proliferation, but expression signatures for CD4+ cells, including high expression of Ki67, suggested a more robust, proliferative trend.
Leveraging patient PBMCs taken at the 9.3 year time point, the authors then used single cell T-cell receptor (TCR) sequencing and combined single cell RNA sequencing with cell surface protein profiling (CITE-seq) to gain additional unbiased insights into the clonality, as well as the transcriptomic and proteomic signatures, that defined these later CD4+ CAR T populations. CAR T cells had significantly reduced clonal diversity compared to normal T cells, and the top 3 clonotypes constituted more than 90% of CAR T cells. Additionally, CAR T cells displayed transcriptomic features of ongoing proliferation, including expression of PCNA, MCM6, and TOP2A, among other genes (2).
This CD4+ compartment, emerging as the dominant CAR T-cell population in the context of long-term remission, also had some unconventional qualities—namely, cytotoxicity. CD4+ cells were marked by significant upregulation of genes encoding cytotoxic enzymes, including GZMK and GZMA. CITE-seq data also showed upregulation of activation markers CD38 and HLA-DR; likewise, transcriptomic data showed upregulation of genes encoding for cytokines interleukin 10 (IL-10) and IL-32, indicators of functional activity among CAR T cells. Together, this data suggests that long-persisting CD4+ CAR T cells were functionally active, not exhausted over time (2).
Identifying a novel CD4-CD8- CAR T-cell population
While the 2010 flow cytometry analysis revealed clear CD4+ and CD8+ compartments, this follow-up study revealed a third unique CD4-CD8- CAR+ population—a silent population that emerged early with CD8+ CAR T cells, hidden because it did not carry these classic cell surface markers. Transcriptomic data from patient PBMCs taken at month 3 and year 3 confirmed a double-negative cell cluster with reads mapped to the 5′ CAR construct, expression of TRDV1 and TRGV4, and lack of TCRαβ clonotypes. Thus, these cells were annotated as gamma delta (γδ) CD4-CD8- CAR T cells.
The presence of this other CAR subpopulation, with a potentially flexible phenotype shifting between CD4, CD8, and gamma-delta T cells, could contribute additional longevity to the living therapy—“Multiple cells can have effects, and maybe more than one is better” (Dr. Marcela Maus; 3).
Successful therapies need cellular insights
This study adds valuable insights to our understanding of the evolution of CAR T cells over time, and represents a key step forward in our approach to cancer treatment. Long-term remission is an essential precursor to that ever elusive end goal of cancer research—cure. With these examples of successful treatment to learn from, there is hope that we can continue to improve current therapies and make long-term remission a more frequent outcome for cancer patients.
This is also a story of technological evolution. Advancements in single cell multiomic profiling techniques have opened the door to studying CAR T cells with higher resolution and broader coverage than ever before. While we knew this treatment worked, with the aid of powerful technology, the “how” is becoming increasingly clear.
To read more about this study, review the publication here. And explore 10x Genomics resources for cancer research.
References:
- Johnson C. “One Big Myth about Medicine: We Know How Drugs Work.” The Washington Post, WP Company, 25 Nov. 2021, https://www.washingtonpost.com/news/wonk/wp/2015/07/23/one-big-myth-about-medicine-we-know-how-drugs-work/.
- Melenhorst J, et al. Decade-long leukaemia remissions with persistence of CD4+ CAR T cells. Nature 602: 503–509 (2022). doi: 10.1038/s41586-021-04390-6
- Chen A. “Researchers Label Early CAR-T Therapy Patient 'Cured' after Living a Decade without Cancer.” STAT, 7 Feb. 2022, https://www.statnews.com/2022/02/02/cart-cancer-therapy-leukemia-treatment/.
- https://www.cancer.gov/about-cancer/treatment/research/car-t-cells
- Kalos M, et al. T Cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3: 95ra73 (2011). doi: 10.1126/scitranslmed.3002842
- Sallusto F, et al. Central memory and effector memory T cell subsets: Function, generation, and maintenance. Annu Rev Immunol 22: 745–763 (2004). doi: 10.1146/annurev.immunol.22.012703.104702
