Bacterial single cell RNA-seq reveals antibiotic resistance mechanisms
*Impact at a glance: Bacterial heterogeneity is a crucial matter of public health because of its relevance to antibiotic resistance. Now, a customer-developed method called BacDrop overcomes the limitations of bulk analysis, allowing researchers to perform highly scalable single cell analysis of bacterial cells and opening up a new world of research. Method developers from the Broad Institute of MIT and Harvard and Massachusetts General Hospital tested BacDrop against Klebsiella pneumoniae clinical isolates, characterizing bacterial heterogeneity and variable responses to different antibiotic treatments.*
The Innovator Blog Series celebrates research conducted by 10x Genomics customers who have demonstrated scientific ingenuity by adapting Chromium Single Cell or Visium Spatial sequencing-based assays. These innovations are distinguished by their originality and potential impact on scientific discovery, including providing access to novel analytes, advancing multiomic analysis techniques, and demonstrating critical applications to human health and disease research. These techniques are customer developed, meaning they are not officially supported by 10x Genomics.
Why single cell for bacteria
Whatever your perception of bacteria may be, these tiny, squirming, ciliated buggers are, nonetheless, cells. They have a transcriptome, too, which can be measured (now, at the single cell level) to understand cellular identity and cell-to-cell heterogeneity.
Bacterial heterogeneity is a crucial matter of public health because of its relevance to antibiotic resistance. According to a 2019 global analysis of antimicrobial resistance, 1.27 million people died as a direct result of drug-resistant infections, far more than HIV/AIDS or malaria, which took 864,000 lives and 643,000 lives, respectively (1,2). As staggering as these numbers already are, some estimates project that by 2050, as many as ten million people could die each year because of antimicrobial resistance (2).
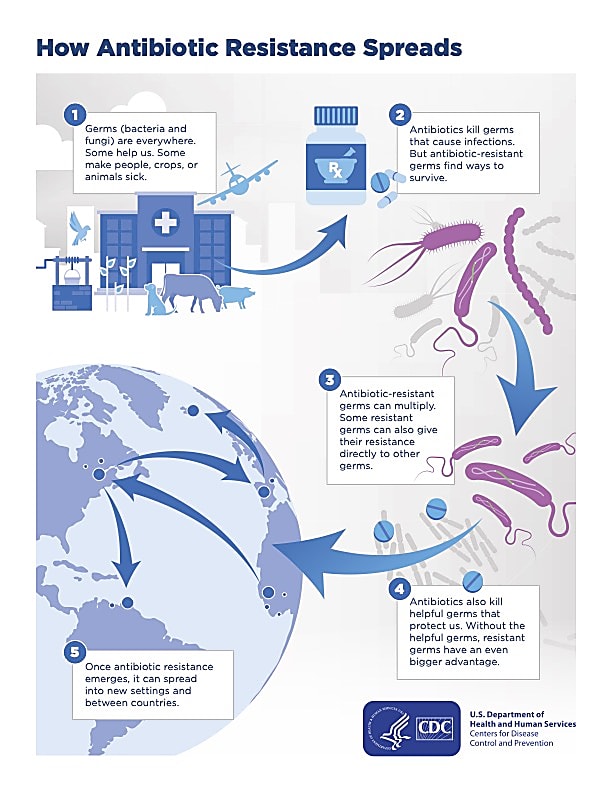
Bulk RNA sequencing is the most prevalent method to sequence the bacterial transcriptome; however, it can be easy to miss subtle cell-to-cell heterogeneity and rare cell subtypes as readouts from this method provide an average of gene expression in a sample. For this reason, characterizing bacterial cell heterogeneity and responses to antibiotics at the single cell level could be valuable in identifying persister populations and characterizing the underlying mechanisms of antibiotic resistance.
This motivated researchers from the Broad Institute of MIT and Harvard and from Massachusetts General Hospital to develop a method built on the Chromium Single Cell platform that enables highly scalable single cell analysis of bacterial cells. They used their technique, called BacDrop, to characterize Klebsiella pneumoniae clinical isolates and define the variable responses this population of bacteria has to different antibiotic treatments (3).
Keep reading to explore their innovative methods, which overcome a number of challenges to accessing mRNA in bacterial cells, and offer new insights into the role of mobile genetic elements in driving antibiotic resistance.
In-cell reactions solve the mRNA access problem
While eukaryotic cells have been tested and validated extensively on single cell sequencing platforms, prokaryotic cells (in this case, bacteria) don’t follow the eukaryotic norms. For example, one crucial difference between mammalian and bacterial cells is in the composition and accessibility of their messenger RNA (mRNA) molecules, or transcripts. These molecules carry the coding sequences for protein synthesis (4) and are quantified in gene expression profiling experiments.
Bacterial cells are also heavily dominated by ribosomal RNA (rRNA) and genomic DNA (gDNA). Accessing bacterial mRNA is also difficult because their cell walls require harsher lysis conditions than most eukaryotic cells, which risks damaging RNA integrity (3).
The solution to these two challenges go hand-in-hand. The research team from the Broad Institute developed a sample preparation method by which they fixed bacterial cells with formaldehyde—in essence freezing the cells and their contents—then permeabilized the cells with lysozyme (an enzyme that lyses bacterial cell membranes) while using RNase Inhibitor to maintain RNA integrity. After washing and resuspending the permeabilized cells, the team treated them with a series of enzyme solutions to perform in-cell bacterial rRNA and gDNA depletion (3).
Continuing their approach of performing reactions inside the fixed cells, the team developed a strategy to attach poly-A tails to bacterial mRNA. First, they plated cells in 384- or 96-well plates containing unique molecular identifier (UMI) sequences, round 1 plate barcodes to label cells, and a reverse transcription (RT) reaction solution. This reaction generated complementary DNA (cDNA) copies of all the mRNA molecules inside each bacterial cell with a plate-specific barcode.
Recovering and pooling the cells, the team then performed another in-cell reaction to “tail” cDNA molecules using terminal transferase, which involves the addition of deoxynucleotides to the 3' end of bacterial transcripts since they lack poly-A tails. Now, all cDNA molecules would have a poly-A tail, making it possible to perform second-strand cDNA synthesis using oligo-dT primers (a cDNA enrichment step that happens inside droplets on the Chromium platform), and subsequently to attach a round 2 droplet barcode to double-stranded cDNA. Using this approach, each unique cell would be identified by the combination of the round 1 plate barcode and round 2 droplet barcode, allowing researchers to load each droplet with multiple cells and, therefore, increase the throughput of the assay (3).
If you thought all of that was cool, here, the true wildcard of their experiment: the team prepared cells for droplet generation on the Chromium platform, but specifically using the Single Cell ATAC Library and Gel Bead Kit. This kit is technically designed to barcode DNA fragments from single nuclei. However, in this case, the researchers hacked it to capture cDNA fragments from single bacteria cells (pause, as your mind is blown). Using this high-throughput, microfluidics platform—including hundreds of thousands of unique droplet barcodes provided in the single cell ATAC kit—allowed the team to reduce plate-based combinatorial index steps and increase the efficiency and scale of their single cell analysis workflow.
After running bacterial cells through the assay, the team purified and enriched cDNA content, then constructed sequencing-ready libraries, completing an innovative and unexpected experiment to capture and quantify the bacterial transcriptome at single cell resolution.
BacDrop overcomes bulk RNA-seq to reveal a key factor of bacterial cell heterogeneity
Putting BacDrop to the test was next on the researchers’ agenda—in particular, assessing its ability to distinguish bacterial heterogeneity at the transcriptional level between different species, within the same population, and under different antibiotic treatment conditions (3).
The team first validated BacDrop by successfully distinguishing four different bacterial species—K. pneumoniae, P. aeruginosa, gram-negative E. coli, and gram-positive E. faecium—in single cell RNA-seq data. They also tested the molecular sensitivity and coverage of their method by generating a one-million-cell library of K. pneumoniae, sequenced at 5,000 reads per cell. They recovered 60,000 cells with at least 15 mRNA genes per cell and, combining analysis of all cells, detected expression for 96% of the genes in the entire genome, which validated BacDrop’s sensitivity (3).
Their next experiments honed in on the identification of heterogeneous subpopulations within a cultured clinical isolate of K. pneumoniae MGH66 under antibiotic-treated and untreated conditions. One MGH66 culture was split into four cultures: one was left untreated, while the remaining three were treated with antibiotics (meropenem, ciprofloxacin, and gentamicin) of varying mechanisms of action. Each of the four samples were first barcoded with round 1 plate barcodes separately, then pooled together for single cell analysis. Cell clustering was dictated by the unique gene expression changes induced by each of the antibiotic treatment conditions, confirming BacDrop’s ability to disentangle both heterogeneous populations within the same bacterial species and transcriptional programs induced by treatment.
Bulk RNA-seq was performed in parallel on biological replicates sharing the same treatment scheme and, generally, bulk data showed alignment to the gene expression changes seen in single cell analysis of the ciprofloxacin- and gentamicin-treated samples. However, this alignment diverged in the meropenem sample: bulk RNA-seq showed minimal transcriptional changes as a result of treatment; however, single cell analysis revealed four subpopulations with distinct molecular responses characterized by upregulation of genes involved in the stress response, cell wall synthesis, DNA replication, and cold shock response (3).
Bulk RNA-seq also could not reveal a small but important population within the untreated K. pneumoniae sample. The team identified two major subpopulations by single cell analysis: a large homogeneous subpopulation and a much smaller subpopulation (comprising about 4.5% of cells) showing increased expression of the IS903B transposase gene, a mobile genetic element (MGE) that can move around and duplicate itself (and has 83 times) within the MGH66 genome (3). This small MGE population could explain the K. pneumoniae strain’s tendency to acquire antibiotic resistance (3).
To test this hypothesis, the research team used BacDrop to study a carbapenem-resistant K. pneumoniae clinical isolate (BIDMC35). This class of bacteria are a serious threat to public health, because, in some cases, they are resistant to all available antibiotics (5). Single cell analysis of 9,748 bacteria cells again revealed MGE-driven subpopulations: in this case, three unique cell clusters defined by expression of three different transposon genes, IS4321 family transposase (195 cells, 2%), insH transposase (146 cells, 1.5%), and IS110 family transposase (133 cells, 1.4%). With this high-resolution view of rare subpopulations, the team confirmed the role of MGEs in driving heterogeneity and likely in antibiotic resistance as well (3).
Transforming microbiology with cellular resolution
What could microbiologists and infectious disease researchers do with full access to the bacterial transcriptome at single cell resolution? BacDrop offers a tool to answer that and opens a door to a new world of research. Scientists are empowered to delve deeper into the biological heterogeneity of pathogenic bacterial species, environment species, and even the microbiome—and to understand how antibiotic treatments work, or why they don’t. Given this huge potential, BacDrop is fully qualified to stand among the other customer-developed methods featured in the Innovator Series.
Want to read more Innovator Series posts? Learn about a method to measure viral RNA in single cells and in spatially resolved tissue sections here.
Looking for other infectious disease resources? Check out our page!
References:
- Murray C, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. The Lancet 399: P629–655 (2022). doi: 10.1016/S0140-6736(21)02724-0
- Thompson T. The staggering death toll of drug-resistant bacteria. Nature (2022). doi: 10.1038/d41586-022-00228-x
- Ma P, et al. Bacterial droplet-based single-cell RNA-seq reveals antibiotic-associated heterogeneous cellular states. Cell 186: 1–15 (2023). doi: 10.1016/j.cell.2023.01.002
- Ribosomes, Transcription, and Translation. Scitable by Nature Education (2014).
- https://www.cdc.gov/hai/organisms/cre
About the author:

