Single cell insights into an Alzheimer’s disease drug target
*Impact at a glance: The Alzheimer’s Association estimates that 55 million people worldwide are affected by Alzheimer’s disease (AD) or another form of dementia. This blog highlights the incredible research that a group of neuroscientists from institutes across California published, detailing a method built on the Chromium Single Cell platform to isolate and profile cells with cytoplasmic neurofibrillary tangles and thereby accelerate biomarker discovery in neurodegenerative disorders (1).*
What a tangled web AD weaves
In 2019, Researchers reported that over 200 investigational programs seeking the development of new treatments for AD failed or were abandoned (2). Critics cite numerous reasons for the nearly 99% fail rate of AD clinical trials—improper doses, treatment initiation late in disease progression, etc.
But a major issue is our lack of understanding of how the pathological hallmarks of AD actually drive disease symptoms, as evidenced by recent controversy over the first FDA-approved drug that targets the underlying pathology of the disease—Aduhelm (3).
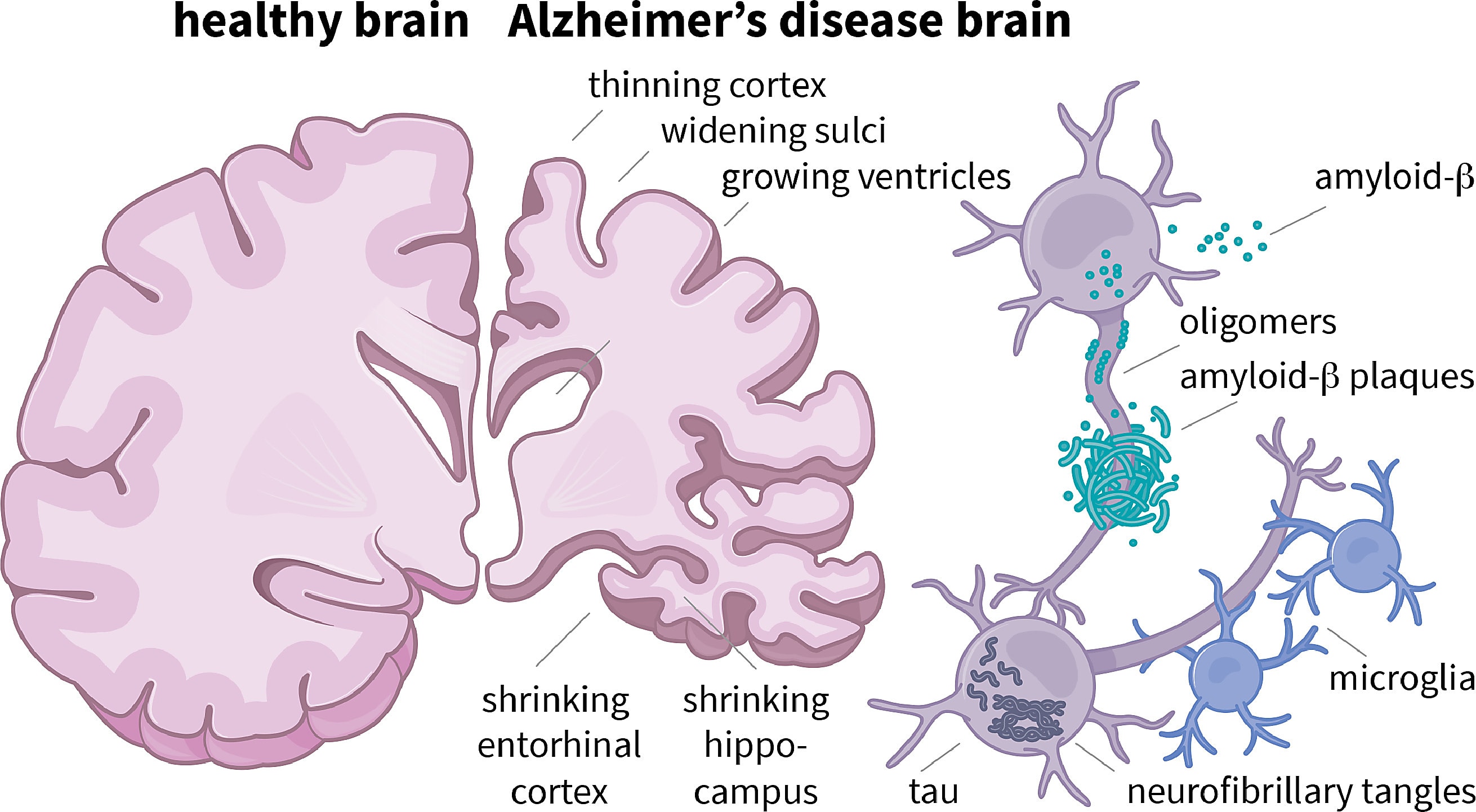
In 2021, the US Food and Drug Administration (FDA) made headlines with its controversial, accelerated approval of Aduhelm, an antibody that targets and subsequently reduces the amyloid-beta plaques that build up in AD-affected brains early in the disease. It was the first drug for AD approved since 2003.
The approval of a drug that many argued demonstrated limited efficacy in clinical trials sparked discussion about why so many clinical trials testing drugs to treat AD fail, and whether or not amyloid-beta plaques are the best therapeutic target for treating the disease (5).
Many are still pursuing amyloid plaques as a therapeutic target—another antibody targeting amyloid plaques was approved by the FDA in January despite safety concerns (6,7). But others are turning to the protein tau, a component of the neurofibrillary tangles (NFTs) that form in neurons during the later stages of AD and become more abundant as the disease progresses (8).
Researchers have struggled to effectively target intracellular tau (9), but results of a recent phase IIb clinical trial testing the efficacy of a new antisense oligo in 46 AD patients saw a reduction in tau levels (10).
But researchers can’t reach a consensus on whether NFTs protect against or promote the high levels of neuronal cell death often observed in patients with AD, leaving an important question: will pharmaceutically inhibiting the formation of NFTs have any clinically relevant effect on disease progression?
Untangling tightly wound neuronal fibers with single cell RNA-sequencing
Previous studies employed bulk RNA-seq to identify NFT-affected neurons and signaling pathways. But heterogeneous cellular and transcriptional responses in the brain can be highly cell-type-specific, which bulk sequencing can’t capture. A group of researchers recently published a study in Neuron that employed single cell RNA-sequencing (scRNA-seq) to wade through the heterogeneity and uncover the effects of NFT-formation on atrophied brains from AD patients (1).
But they had to overcome a major obstacle—differentiating between neurons with and without NFTs in frozen patient brains. Researchers often isolate nuclei rather than cells in frozen patient samples since cell membranes can be pierced by ice crystals during freezing, but NFTs are intracellular and therefore not captured by nuclei isolation.
To capture both nuclei and NFTs, the researchers isolated somas, which contain nuclei and NFTs. Using fluorescence-activated cell sorting (FACS), they isolated NFT-containing somas by immunostaining cells with the monoclonal antibody AT8, which recognizes hyperphosphorylated tau protein. About 7% of all neurons from each histological section used for scRNA-seq contained NFTs.
They ultimately isolated 63,110 somas with or without NFTs from the prefrontal cortex of frozen late-stage-AD donor brains and 57,534 somas from age-matched controls. First performing unsupervised clustering of neurons from AD brains without NFTs, they determined that, despite significant atrophy in donor brains, all major neuronal types were present in the samples. But they quickly discovered that a higher percentage of certain subpopulations of both excitatory and inhibitory neurons were AT8 positive.
They performed differential gene expression (DGE) analysis between neurons with and without NFTs to identify potential transcriptional signatures of NFT susceptibility. Since hyperphosphorylated tau is implicated in chromatin remodeling, the authors were not surprised to find that most differentially expressed genes were upregulated in neurons with NFTs.
They further analyzed their data with the online analysis platform SynGO, a curated collection of over 1,000 validated synaptic gene annotations and ontologies (11). They identified three well-established biomarkers of neurodegeneration and cognitive decline and fifteen known risk factors of AD.
But NFT-bearing neuronal populations were not more sensitive to cell death—there was no decrease in the amount of any neuronal populations in atrophied AD brains compared to healthy control brains. However, they did find that ATF4, a cellular stress response protein that contributes to returning metabolically malfunctioning cells to homeostasis, was highly upregulated in NFT-bearing cells.
Moving towards a treatment
The researchers ultimately developed a method for profiling cells with cytoplasmic neurofibrillary tangles, providing others a platform to explore the transcriptional changes driving other tauopathies such as frontotemporal dementia and progressive supranuclear palsy.
But they weren’t able to use their biomarker discovery platform to fully parse the role NFTs play in neurodegeneration in AD.
“NFTs may represent cell-type specific responses to cellular and microenvironmental stressors in AD and that these responses are neither fully protective nor deadly to the cells bearing them,” wrote the authors.
Their method does, however, offer yet another tool to assist in identifying the next putative drug target for neurodegenerative diseases like AD—and to help unravel the mechanistic underpinnings of this debilitating disease.
Discover how our suite of single cell and spatial technologies can help you unravel the inner workings of neurodegeneration in this application note.
References:
- Otero-Garcia M, et al. Molecular signatures underlying neurofibrillary tangle susceptibility in Alzheimer’s disease. Neuron 110: P2929–2948 (2022). doi: 10.1016/j.neuron.2022.06.021
- Yiannopoulou KG, et al. Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 7: 97 (2019). doi: 10.3390/biomedicines7040097
- https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug
- Schafer A, et al. Predicting brain atrophy from tau pathology: a summary of clinical findings and their translation into personalized models. Brain Multiphysics 2: 100039 (2021). doi: 10.1016/j.brain.2021.100039
- https://www.nytimes.com/2021/07/19/health/alzheimers-drug-aduhelm-fda.html
- https://www.science.org/content/article/fda-approves-new-antibody-slow-alzheimer-s-disease-even-safety-concerns-linger
- Van Dyck CH, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med 388: 9–21 (2023). doi: 10.1056/NEJMoa2212948
- Braak H and Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 138: 2814–2833 (2015). doi: 10.1093/brain/awv236
- Congdon EE and Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 14: 399–415 (2018). doi: 10.1038/s41582-018-0013-z
- Mummery CJ, et al. Tau-targeting antisense oligonucleotide MAPTRx in mild Alzheimer’s disease: a phase 1b, randomized, placebo-controlled trial. Nat Med (2023). doi: 10.1038/s41591-023-02326-3
- Koopmans F, et al. SynGO: An Evidence-Based, Expert-Curated Knowledge Base for the Synapse. Neuron 103: P217–234 (2019). doi: 10.1016/j.neuron.2019.05.002
