Single cell RNA-seq identifies putative biomarker to distinguish responders and nonresponders to adoptive cell therapy in metastatic melanoma
*Impact at a glance: Using single cell sequencing to analyze melanoma samples before and after treatment, the study uncovered shifts in immune cell populations that linked with positive therapeutic outcomes. These insights show how the immune system adapts during successful treatment and point to potential biomarkers that could be developed to predict which patients are most likely to respond favorably.*
In this blog, we discuss how researchers performed comprehensive single cell analysis of pre- and post-treatment tumor biopsies from metastatic melanoma patients enrolled in a phase 1 clinical study of tumor-infiltrating lymphocyte adoptive cell therapy (TIL-ACT). The results of the study, published in Science Immunology* in February, demonstrated baseline and post-treatment immune cell states and cell–cell interactions within the tumor microenvironment underlying positive response to therapy, and pointed to a possible biomarker for improved patient selection for TIL-ACT (1). Find more details as we break down the clinical trial goals and results below.
Phase 1: Adoptive cell therapy success driven by T-myeloid cell network in the tumor microenvironment
Cancer type: Metastatic melanoma
Therapy: Adoptive cell therapy (ACT) using ex vivo–expanded tumor-infiltrating lymphocytes (TILs), also known as TIL-ACT, in combination with high dose interleukin-2 (IL-2) depending on patient tolerance, and subsequently followed by nivolumab (anti-PD1 monoclonal antibody) treatment for eligible patients.
Trial aim: Define the feasibility and safety of TIL-ACT in metastatic melanoma patients, with a secondary objective of defining the feasibility and safety of nivolumab rescue following TIL-ACT.
Leading institutions: Ludwig Institute for Cancer Research, University of Lausanne; Lausanne University Hospital; Agora Cancer Research Center; Center for Cell Therapy, CHUV-Ludwig Institute
Pharmaceutical collaborators: Bristol Myers Squibb
ClinicalTrials.gov registration: NCT03475134
Clinical results: 13 patients with metastatic melanoma who had shown disease progression on immune-checkpoint blockade (ICB) therapies received TIL-ACT and intravenous IL-2 support after lymphodepletion chemotherapy. The research team observed objective responses in six patients, thus 46% of analyzed patients were denoted responders. Two of these patients showed an ongoing, complete response, while four had a partial response. Nonresponders were classified as those patients who had progressive disease (four) and those with stable disease (three). Median progression-free survival among the 13 patients was 5.6 months, and median overall survival was 8.8 months. Of note, both complete response patients received mono-ICB therapy prior to TIL-ACT, while the four partial response patients and six of the seven nonresponders received dual ICB therapy prior to this trial.
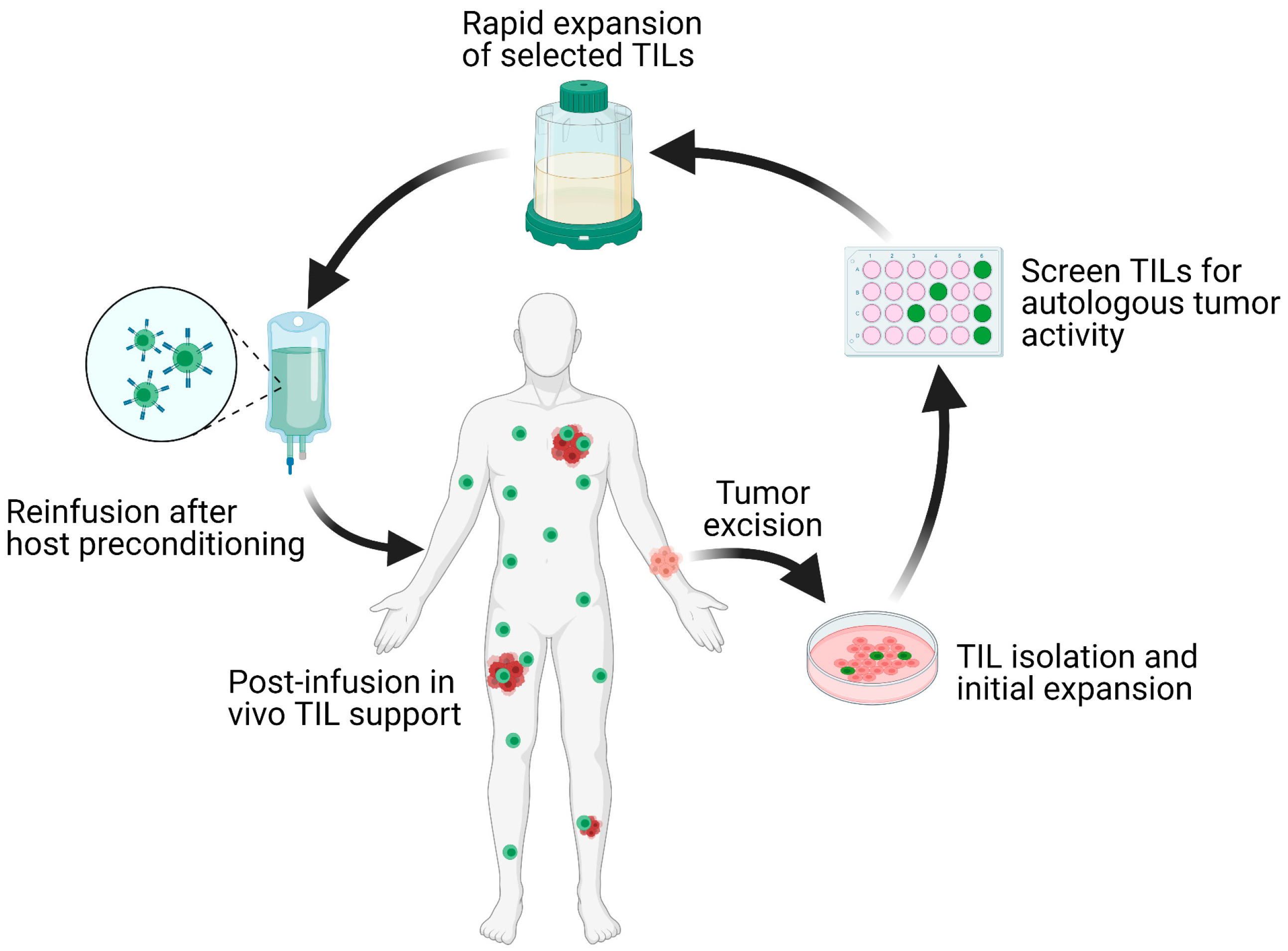
What is tumor-infiltrating lymphocyte adoptive cell therapy?
This therapy utilizes a patient’s endogenous T cells that have successfully infiltrated a tumor and, thus, have broad antigen recognition in tumor cells. TILs may become ineffective as a result of an unfavorable tumor microenvironment enriched with suppressive regulatory T cells or myeloid suppressor cells; however, this therapeutic approach allows scientists to isolate TILs from a harvested tumor, expand the cells ex vivo, then reinfuse the cells back into the patient. Lymphodepletion chemotherapy regimens typically precede TIL-ACT with the goal of removing regulatory T cells and cytokine sources from the tumor microenvironment that can inhibit TILs’ anti-tumor immunity and proliferation potential (2).
Why was TIL-ACT considered for these patients? What additional advantages does TIL-ACT offer in situations where immune-checkpoint blockade therapies are no longer effective?
Researchers from the Ludwig Institute for Cancer Research, in partnership with scientists at the CHUV-Ludwig Institute and Lausanne University Hospital, explored TIL-ACT in this clinical trial because of its demonstrated efficacy in patients with metastatic melanoma in prior clinical studies (1). A legacy of efficacy, albeit in certain patients, dates back to 1988, when researchers saw a 55% objective response rate in the first in-human test of tumor-infiltrating lymphocyte adoptive cell therapy for metastatic melanoma (2). Notably, a 2020 clinical study of TIL-ACT in melanoma patients whose cancer had progressed after PD-1 blockade therapy or treatment with a BRAF±MEK inhibitor showed additional positive responses, including 36.4% objective response rate and a disease control rate of 80%. In this case, a majority of patients who responded to the adoptive cell therapy had progressed after ICB, suggesting that the two immunotherapies are not redundant. TIL-ACT may offer an advantage over ICB therapies in that ICB relies on the presence of tumor-reactive T cells in the tumor microenvironment; however, these crucial cells could become dysfunctional or may not be present at the necessary abundance (or at all). In contrast, TIL-ACT allows selection and reintroduction of tumor antigen-specific T cells, which may be able to overcome dysfunction-inducing triggers in the tumor microenvironment in combination with IL-2 co-culture and lymphodepletion tactics (2).
Applications of single cell sequencing
- Establishing baseline tumor-intrinsic characteristics
- Characterizing tumor-infiltrating T-cell populations prior to TIL-ACT
- Defining immune cell crosstalk in the tumor microenvironment
- Defining TIL-ACT mechanism of action
Establishing baseline tumor-intrinsic characteristics
Given the influence of the tumor microenvironment (TME) and innate tumor characteristics on adoptive cell therapy efficacy, the research team sought to establish the baseline cellular landscape for patient tumors using single cell RNA-sequencing. These insights helped them identify tumor-intrinsic factors that influence therapeutic efficacy, as well as biomarkers to distinguish likely responders and nonresponders.
The team first analyzed surgical samples from 10 patients taken prior to TIL-ACT (including 6 responders and 4 nonresponders) by single cell RNA-sequencing. Specifically, resected tumors were dissociated and analyzed by Chromium Single Cell 3’ Gene Expression to produce a UMAP projection of the main cell types and their proportions. Tumor cells notably clustered separately for each sample, pointing to high patient specificity. Using single cell data to infer chromosomal copy number variation in malignant tumor cells, the team observed that responders had more copy number deletions, suggesting these tumor cells had higher genomic instability. Responder tumor cells also showed increased activation of a number of immunogenic programs—including IFN-α and IFN-γ responses, complement activation, antigen presentation, and immune checkpoints—compared to nonresponder tumor cells.
Characterizing tumor-infiltrating T-cell populations prior to TIL-ACT
To further characterize the immune cell composition of the TME, the team isolated CD45+ cells from all 13 patient tumor samples and analyzed them with Chromium Single Cell 5’ Gene Expression and single cell T-cell receptor sequencing (scTCR-seq). This revealed nine unique CD8+ TIL clusters representing varied T-cell states, including naive-like, effector memory, exhausted, and natural killer (NK)–like T cells.
The team aimed to further differentiate responders and nonresponders by understanding the differences between tumor-infiltrating T-cell states from both patient groups in these baseline tumors. They observed enrichment of precursor exhausted (PEX) and exhausted (TEX) CD8+ T cells in responders, which had, among all T-cell states, the highest gene signatures associated with T-cell receptor (TCR) signaling, cytotoxicity, and proliferation, suggesting these populations were actively engaging tumor antigens. PEX and TEX CD8+ T cells in responders also showed signatures of dendritic cell CD28 costimulation, which is a known mechanism for retaining proliferative capacity. In contrast, they observed that CD8+ T cells in nonresponders were overexpressing a viral-specific gene signature, which is indicative of a bystander phenotype.
The team next investigated the specific baseline T-cell gene expression programs associated with positive clinical response or TIL-ACT failure. They found that clinical response was associated with CD8+ TILs exhibiting increased activity of ZNF831 (zinc finger protein 831) and HIVEP1 (zinc finger protein 40), both of which bind to enhancer elements of genes involved in effector and exhaustion programs. TIL-ACT failure, however, was associated with the presence of naive- and memory-like CD8+ TILs at baseline and heightened expression of genes and transcription factors involved in T-cell development and differentiation, which generally reflected a lack of antigen-experienced tumor-reactive T-cell populations.
Complete response and partial response likely also came down to a difference in baseline TIL characteristics. The research team discovered that CD8+ TILs from complete responders had elevated gene expression pathways for mitochondrial potential and function as well as the electron transport chain, suggesting that, compared to TILs from partial responders, these cells had higher metabolic fitness. These qualities could sustain the T cells for ongoing anti-tumor activity in the tumor microenvironment, ultimately resulting in a better long-term clinical response. An analogy could be made to a car; between two cars, though they might be the same model, one has better fitness to operate well over longer distances and under harsher road or weather conditions.
Together, these insights reflect the extreme importance of understanding the state T cells are in within the tumor microenvironment, due to their influence on disease progression and therapeutic response. And, they ultimately recall the reality that patient variability in the cellular composition of the TME is also a major factor in determining therapeutic response.
Defining immune cell crosstalk as an important feature of therapy-responsive tumors
Beyond defining the characteristics of tumor-infiltrating lymphocytes, the team used their comprehensive single cell analysis of the tumor microenvironment to determine if other cell types influenced therapeutic response. Responders had a significantly higher proportion of exhausted TILs relative to macrophages and dendritic cells (myeloid subsets), the latter of which had upregulated expression of transcriptional programs associated with IFN signaling, antigen presentation, and CD28 costimulation. These findings pointed to the possibility that crosstalk between TILs, macrophages, and dendritic cells in the TME could be a contributing factor to the invigoration of TILs and, ultimately, to positive therapeutic response. Inferred cell–cell interactions suggested that responding patients had a significantly higher baseline of immune cell interactions compared to nonresponders, particularly between CD8+ TILs and CD11c+ myeloid cells. These insights could suggest that T cell–myeloid interactions are the basis of establishing a robust population of tumor-reactive TILs and effective TIL-ACT therapy.
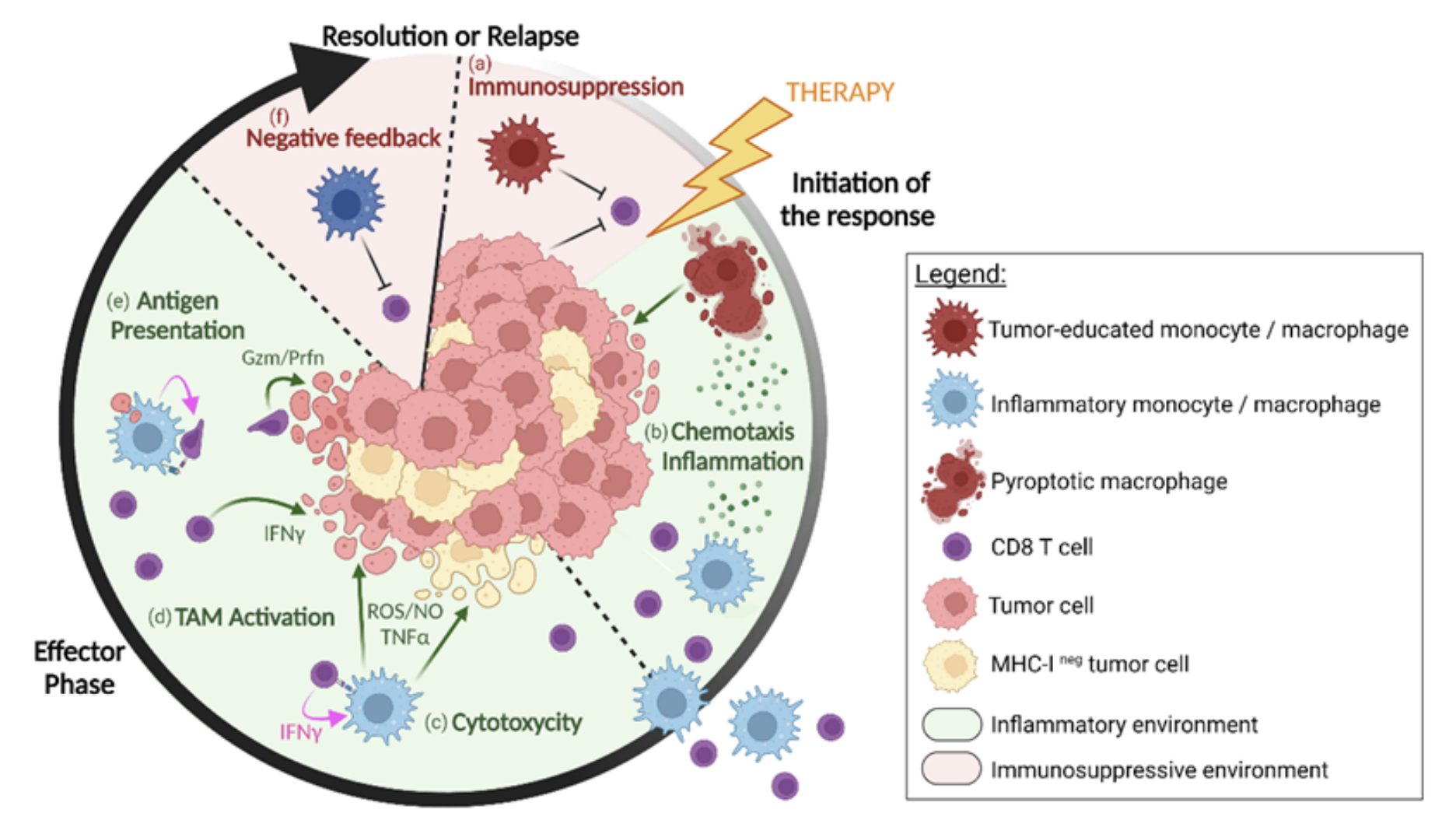
Defining TIL-ACT mechanism of action from post-therapy patient samples
Post-therapy patient samples are equally important to understanding how a treatment may be working as they provide a window into how the therapy changes the composition of the tumor microenvironment, including key immune cell proportions or T-cell states, relative to baseline characteristics. At a minimum of 30 days following TIL-ACT, the research team acquired post-therapy tumor biopsies from 11 patients, which they analyzed with bulk RNA-sequencing using gene expression signatures derived from their single cell data to differentiate major cell types. This analysis showed a clear difference in immune cell composition between responders and nonresponders following therapy: responders reconstituted the crucial CD8+ PEX and TEX T-cell signatures, and showed increased signatures for CXCL9+ and ISG (interferon-stimulated gene) macrophages. In contrast, nonresponders lost these signatures of tumor-reactive T-cell populations.
The team also acquired additional biopsies from seven patients, isolating CD45+ cells, for analysis by single cell 5’ gene expression and scTCR-seq. This revealed that responders showed more heightened predicted interactions between CD8+ T cells and the myeloid subset, particularly CXCL9+ macrophages, after therapy. Again, in contrast, nonresponder post-therapy tumor samples did not show evidence of these immune cell interactions.
Overall, these single cell findings established a mechanism of action for TIL-ACT, suggesting successful therapy may result from the restoration of a tumor-engaging TIL repertoire and a rich network of antigen presenting cells, including CXCL9+ macrophages, in the TME. Additionally, the team’s findings suggest that the baseline presence of exhausted CD8+ TILs and CD11c+ myeloid subsets could serve as a potential biomarker for patient selection.
This study represents an important step forward in the ongoing work to improve cell therapies for solid tumors. In the words of the authors,
“...detailed characterizations of TME states and dynamics as well as cell–cell interactome will help facilitate the development of biomarkers for ACT response and guide the next generation of adoptive cell–based immunotherapies to achieve maximal clinical benefit.”
Read the clinical trial publication.
Additional reading:
Learn more about the technology that supported these clinical findings:
- Chromium Single Cell Gene Expression (3’)
- Chromium Single Cell Immune Profiling (5’ gene expression and scTCR-seq)
Find more incredible studies and researcher stories about cell and gene therapy for cancer treatment using 10x Genomics technology:
- Behind the clinical trial: Advancing CAR T-cell therapies for kids with fatal brain cancer
- Time plus technology reveal secrets of long-lasting CAR T therapy
- A movement rises to guide CAR T-cell therapy development with single cell multiomics
- Single cell immune profiling fuels biomarker discovery in clinical cancer samples
Related blogs:
Improving the success rates of clinical trials requires the right trial design and often includes defining biomarkers that can refine patient populations, clarify drug efficacy and safety profiles, and ultimately guide future drug development efforts.
In our Clinical trials to watch blog series, we feature innovative applications of single cell multiomics and spatial gene expression to clinical samples across trial phases. Follow the series to learn how our tools enable researchers to uncover rare cell populations predictive of therapeutic responses, biomarkers to improve patient stratification, or novel mechanisms of action.
References:
- Barras D, et al. Response to tumor-infiltrating lymphocyte adoptive therapy is associated with preexisting CD8+ T-myeloid cell networks in melanoma. Sci Immunol 9: eadg7995 (2024). doi: 10.1126/sciimmunol.adg7995
- Kirtane K, et al. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer 9: e002723 (2021). doi: 10.1136/jitc-2021-002723
About the author:

