Single cell tools probe deeper into the function of muscle-resident glial cells in response to nerve injury
Though considered rare diseases, neuromuscular disorders and related conditions like ALS are a pressing clinical concern—they range from mildly debilitating to devastatingly fatal, and there are only a handful of approved therapies. In the field of regenerative medicine as it pertains to muscles and the nerve cells that communicate with these tissues, scientists have made strides in understanding the function of muscle satellite cells—stem cells activated after muscle damage or in chronic degenerative conditions. However, less is known about how these cells are regulated in conditions of injury or disease. In this blog post, we take a look at a recent publication using single cell tools to reveal exactly which cells play a role in maintaining the neuromuscular junction after nerve injury.
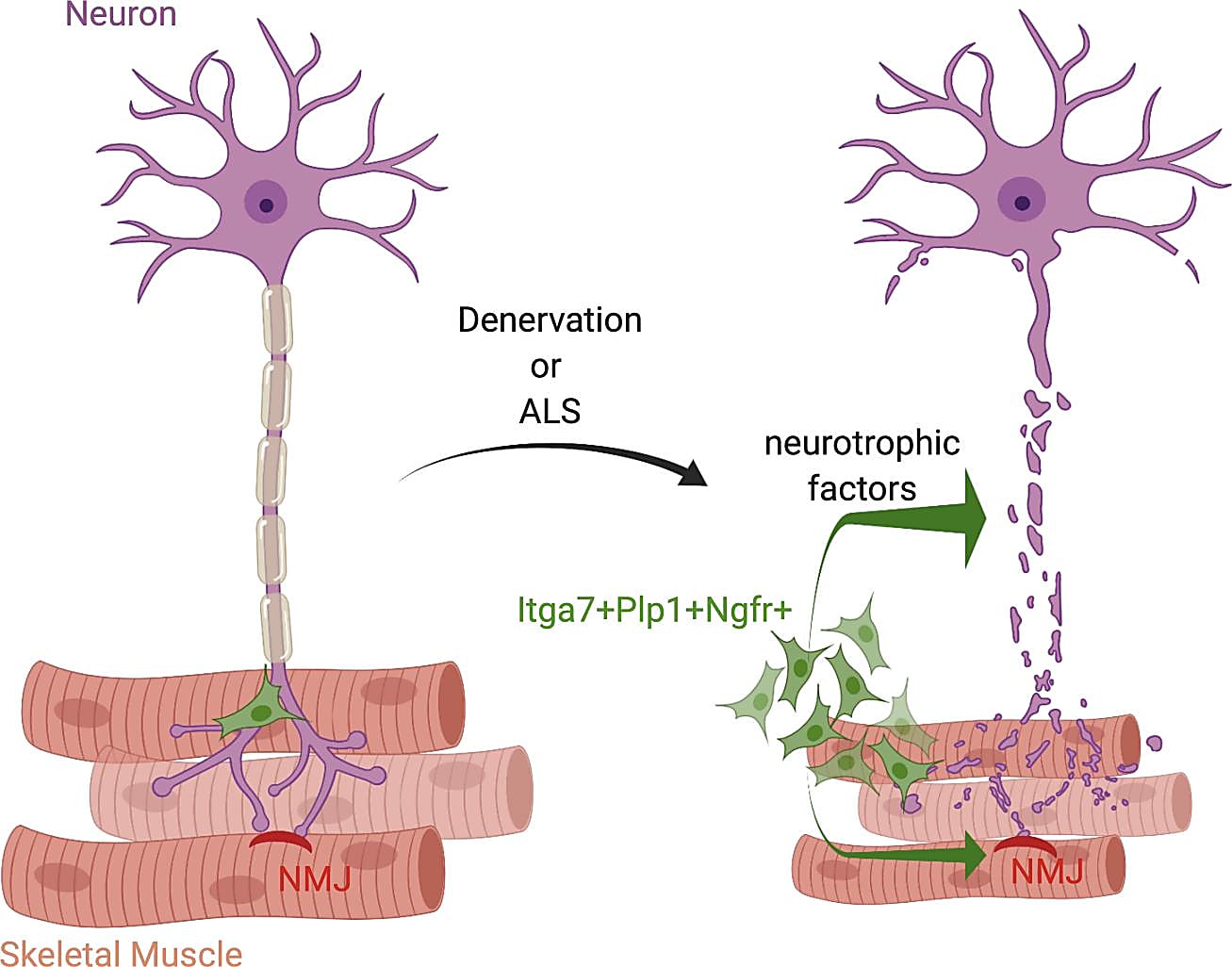
A motor neuron communicates with a muscle fiber by way of the neuromuscular junction (NMJ), which is a synapse that transmits a signal to the muscle fiber and causes the muscle to contract. Recent studies have revealed that, in addition to a large network of muscle-resident cells that respond to muscle or nerve injury, muscle satellite cells (MuSCs) may also help maintain the integrity of the NMJ after nerve injury (1). Other studies have seen changes in MuSCs and fibroadipogenic progenitors in neuromuscular disorders like amyotrophic lateral sclerosis (ALS), where there is progressive loss of NMJ integrity (2).
Healthy muscle–nerve crosstalk is essential in preventing muscle atrophy and nerve loss after traumatic injuries, as well as in the maintenance of the NMJ in chronic neuromuscular disorders. Identifying cell types and functional pathways involved in nerve injury will play a key role in the future development of therapies that target loss of muscle function due to denervation. In a recent publication led by Daisy Proietti, PhD, then at the Santa Lucia Foundation in Rome and now at the San Raffaele Hospital in Milan, scientists used single cell transcriptomic profiling to reveal a subpopulation of muscle-resident glial cells that respond to nerve injury and may potentially play a role in repairing the NMJ (3).
Characterizing muscle cells that contribute to neuromuscular junction repair
Previous studies have shown that Itga7+ MuSCs help maintain the integrity of the NMJ and aid in its regeneration after nerve injury (2). Itga7 is a cell surface protein commonly used to identify MuSCs, but it is not known if all Itga7-expressing muscle-resident cells are actually stem cells. Thus, Dr. Proietti and team began their study by performing bulk RNA sequencing (RNA-seq) on a group of Itga7+ cells. They found that most of the up-regulated genes were associated with neuronal growth and repair pathways and that this “neurotrophic” gene program occurs in a fraction of cells that are not actually MuSCs.
To determine which subpopulation within these Itga7+ cells had an activated neurotrophic program after nerve injury, they performed single cell RNA-seq on Itga7+ cells isolated from limb muscles of mice after sciatic nerve crush (a total of 3,949 cells). Clustering analysis identified three major populations: MuSCs, smooth muscle mesenchymal cells (SMMCs), and glial cells.
Muscle-resident glial cells drive nerve repair after injury
Their single cell transcriptomic profiling showed that, while there was a similar number of MuSCs and SMMCs in both healthy and denervated muscle, they found an increase in Plp1+ glial cells in response to nerve injury, which they validated using a mouse model. Plp1 is a known neurotrophic gene, and their analysis revealed that Plp1+ glial cells are characterized by a neurotrophic gene expression signature. Most of the up-regulated genes were the same as those identified in the bulk Itga7+ cells, including Ngfr, Tnc, Gdnf, Runx2, and other genes related to nerve development. Since Ngfr wasn’t expressed in other cell types, they used this gene to specifically identify glial cells. When they saw no significant change in the number of Ngfr-/Itga7+ cells after nerve injury, they concluded that the main cell type responding to nerve damage are muscle-resident glial cells.
Potential to target nerve repair in chronic degenerative conditions
In this study, Dr. Proietti’s use of single cell tools allowed the team to tease out muscle-resident glial cells that are specifically activated toward a neurotrophic phenotype. It follows that these cells may be future drug targets for nerve repair in chronic degenerative conditions such as ALS. With this possibility in mind, the team employed an ALS mouse model to discover that, while Ngfr gene expression and Ngfr+ cells among Itga7+ cells increased with disease progression, other phenotypic markers showed a loss of glial cell response. While this may point to the reason behind the gradual but progressive loss of the NMJ in ALS, future studies should aim to investigate whether muscle glial cells can repair the NMJ and, if so, how they may be leveraged in future therapies for chronic diseases like ALS and others.
References:
- Liu W, et al. Loss of adult skeletal muscle stem cells drives age-related neuromuscular junction degeneration. Elife 6: e26464 (2017). doi: 10.7554/eLife.26464
- Scaramozza A, et al. Skeletal muscle satellite cells in amyotrophic lateral sclerosis. Ultrastruct Pathol 38(5): 295–302 (2014). doi: 10.3109/01913123.2014.937842
- Proietti D, et al. Activation of skeletal muscle-resident glial cells upon nerve injury. JCI Insight 6(7): e143469 (2021). doi: 10.1172/jci.insight.143469
