Clinical trials to watch: Single cell and spatial transcriptomics reveal mechanism of action for antibody that shrinks tumor metastases
*Impact at a glance: In a Phase I clinical trial study of the effects of a monoclonal antibody targeting metastatic endometrial cancer, scientists observed a statistically significant decrease in tumor cells after treatment, as well as a decrease in the epithelial-to-mesenchymal transition (EMT) gene expression signature (typically associated with metastatic expansion). Through single cell and spatial analysis of pre- and post-treatment clinical samples, they validated the impact of the antibody and discovered a potential mechanism of action.*
Successful clinical trials take many forms. Clinical trials that identify a transformative therapeutic with long-term efficacy—as in the case of the first ever CAR T-cell infusions in 2010, where two men with chronic lymphocytic leukemia experienced remission after months (and over the next 10 years)—motivate researchers to keep pursuing the elusive goal of a cure for widespread diseases like cancer. Prolonged patient survival and long-term remission, particularly in the oncology field, are among the most hoped-for trial outcomes.
But even a clinical trial that does not meet its primary endpoints can yield critical biological insights to guide future drug discovery efforts. High-resolution single cell and spatial transcriptomics tools enable researchers to analyze patient samples collected throughout the trial and uncover rare cell populations predictive of therapeutic responses, biomarkers to improve patient stratification, or novel mechanisms of action.
In our Clinical trials to watch blog series, we feature innovative applications of single cell multiomics and spatial gene expression to clinical samples across trial phases. These trials have diverse goals and address different, complex diseases, but together they demonstrate the value of taking new approaches to the study of clinical samples and, with high-resolution insights, the possibility of making strides towards improved human health outcomes.
Follow the series as we release new blogs showcasing how single cell and spatial tools can fit into Phase 1–3 clinical trial research.
Phase 1: An antibody effectively reduces tumor bulk and EMT score
Cancer type: Endometrial carcinoma
Therapy: Netrin-1 blockade using an anti-netrin-1 monoclonal antibody (NP137), a first-in-class single agent
Trial aim: Determine safety and efficacy, and establish recommended Phase 2 dose
Leading institutions: Centre Léon Bérard, Université de Lyon
Pharmaceutical collaborators: Netris Pharma
ClinicalTrials.gov registration: NCT02977195
What is the significance of netrin-1 to cancer development and progression, and to endometrial cancer, specifically? There’s evidence that when netrin-1 binds to its receptors it inhibits p53-dependent apoptosis, which, in the absence of netrin-1 interference, typically induces cancer cell death and suppresses tumor growth (1). The authors of this clinical study also demonstrated in a cohort of 72 human endometrial tumors that netrin-1 is significantly upregulated in endometrial carcinoma (EC), and its main receptor is expressed more in tumor tissue than normal, healthy endometrium (2). Data from a preclinical mouse model of EC and netrin-1 blockade further confirmed that targeting netrin-1 could inhibit tumor progression (2).
Clinical results: 14 patients with advanced or metastatic stage IV endometrial carcinoma (EC) were treated with NP137, once every two weeks, for a range of 2–17 total treatment injections (median 5.5). 8 out of 14 showed stable disease while 1 showed a partial response, with 51.16% reduction in target lesions at 6 weeks and up to 54.65% reduction during the following 6 months (2).
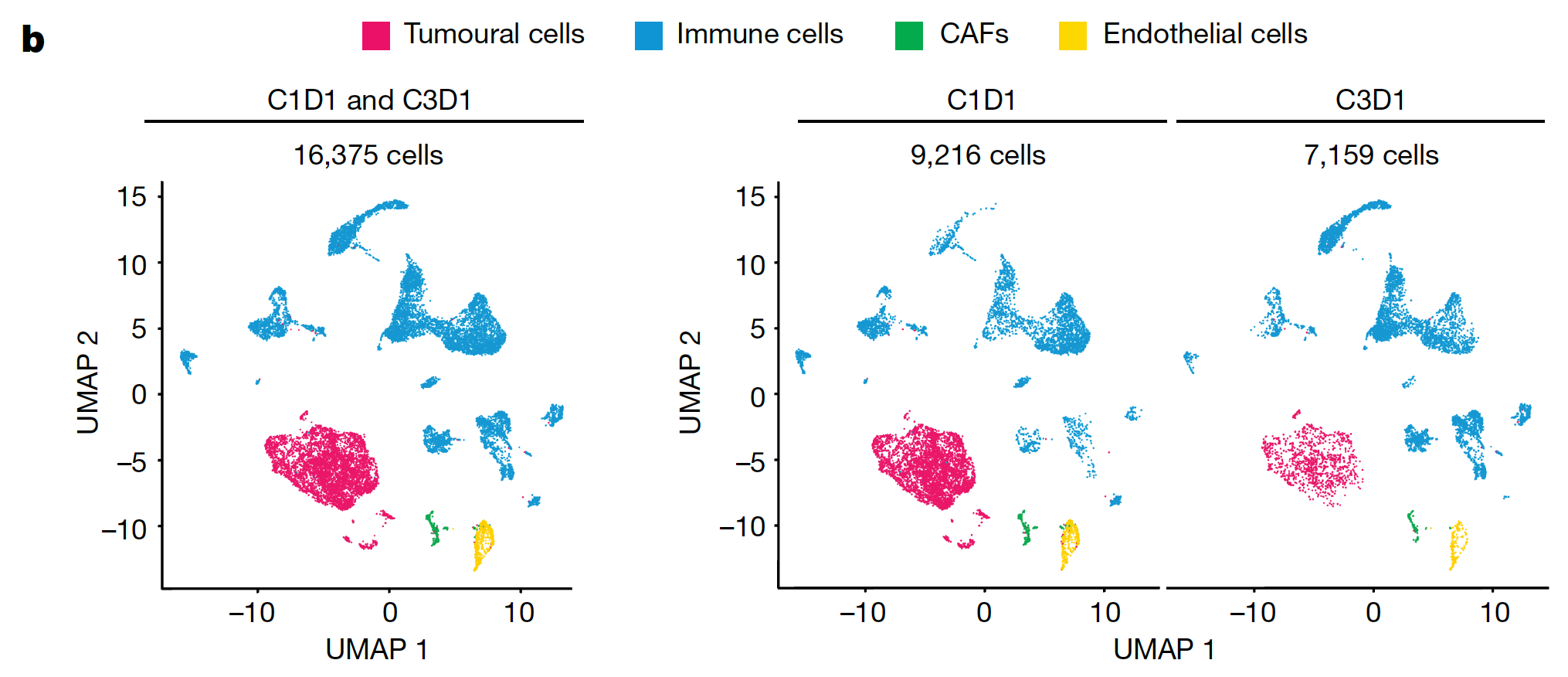
Applications of single cell and spatial sequencing: The research team leveraged pre- and post-treatment (after two cycles of NP137) biopsies of lung metastasis from one trial participant to interrogate the effects of treatment on tumor composition and gene expression. Single cell RNA-seq analysis of biopsies revealed a statistically significant decrease in tumor cells after treatment, as well as a decrease in the epithelial-to-mesenchymal transition (EMT) gene expression signature (typically associated with metastatic expansion (3)), which was also noted in bulk RNA-seq. With the insights enabled by single cell resolution, researchers can confidently identify the cell type–specific events underlying these global gene expression changes, differentiating tumor and non-tumor cellular activity, as well as reveal rare cell subpopulations that can be missed with an average readout.
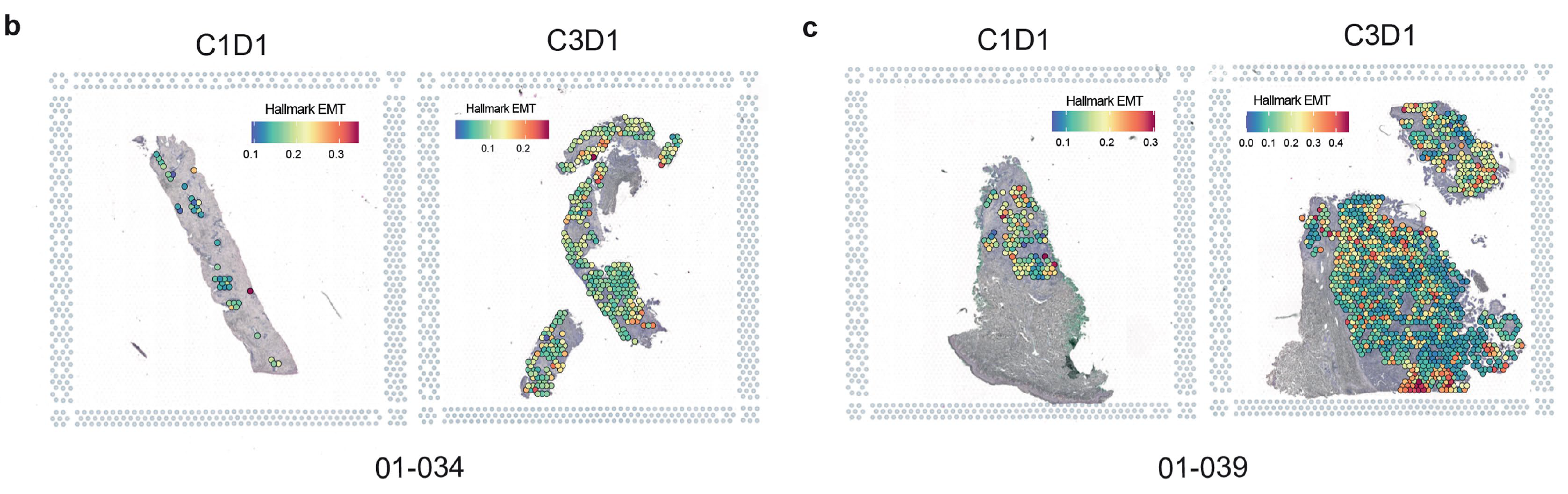
To further confirm EMT gene expression changes seen in bulk data were reflected specifically in cancer cells, the research team leveraged Visium Spatial Gene Expression on FFPE tumor sections from two patients, taken pre- and post-treatment. Spatial data confirmed that EMT signatures decreased after treatment in pathologist-selected tumor regions.
Immune populations were also impacted by NP137 treatment. Single cell data indicated an increase in cytotoxic CD8+ T cells and natural killer cells, while single cell and spatial data both revealed an increase in the number and strength of interactions between T cells and tumor cells (determined by CellChat analysis). This finding built on the evidence for more efficient antigen presentation after NP137 treatment, as noted by a switch from monocytes to dendritic cells interacting with cancer cells (2).
With the insights provided by single cell and spatial transcriptomics, the effects of NP137 can come increasingly into focus. Noting the impact of NP137 on EMT features and the downstream changes in tumor cellular composition and interactions, the authors suggest a potential mechanism of action: NP137, by reducing EMT features, may enhance tumor immune response. There is a current lack of treatment options to alleviate EMT in tumors, but this evidence suggests NP137 could be assessed in combination with other conventional therapies as a way to address that unmet clinical need (2).
Read the full clinical trial publication.
Learn more about the technology that supported these clinical findings:
Download our White Paper to learn how single cell tools can overcome barriers to drug discovery and development.
References:
- Arakawa H. Netrin-1 and its receptors in tumorigenesis. Nat Rev Cancer 4: 978–87 (2004). doi: 10.1038/nrc1504
- Cassier P, et al. Netrin-1 blockade inhibits tumour growth and EMT features in endometrial cancer. Nature 620: 409–416 (2023). doi: 10.1038/s41586-023-06367-z
- Roche J. The epithelial-to-mesenchymal transition in cancer. Cancers (Basel) 10: 52 (2018). doi: 10.3390/cancers10020052
