Single cell tools help decipher transcriptomic landscape of skin-resident innate lymphoid cells in psoriasis
Innate lymphoid cells (ILCs) are innate immune cells that live in tissue and regulate the body’s immune response to pathogens by secreting signaling molecules. When these cells’ behavior goes awry, it can cause allergies, asthma, and autoimmune disorders. While much is known about ILC2s, one type of innate lymphoid cell, it is less understood how ILC3s contribute to the inflammation behind psoriasis. In a recent study, scientists combined single cell gene expression and chromatin state profiling alongside computational analysis to decipher the heterogeneity among skin-resident ILCs in this inflammatory skin condition.
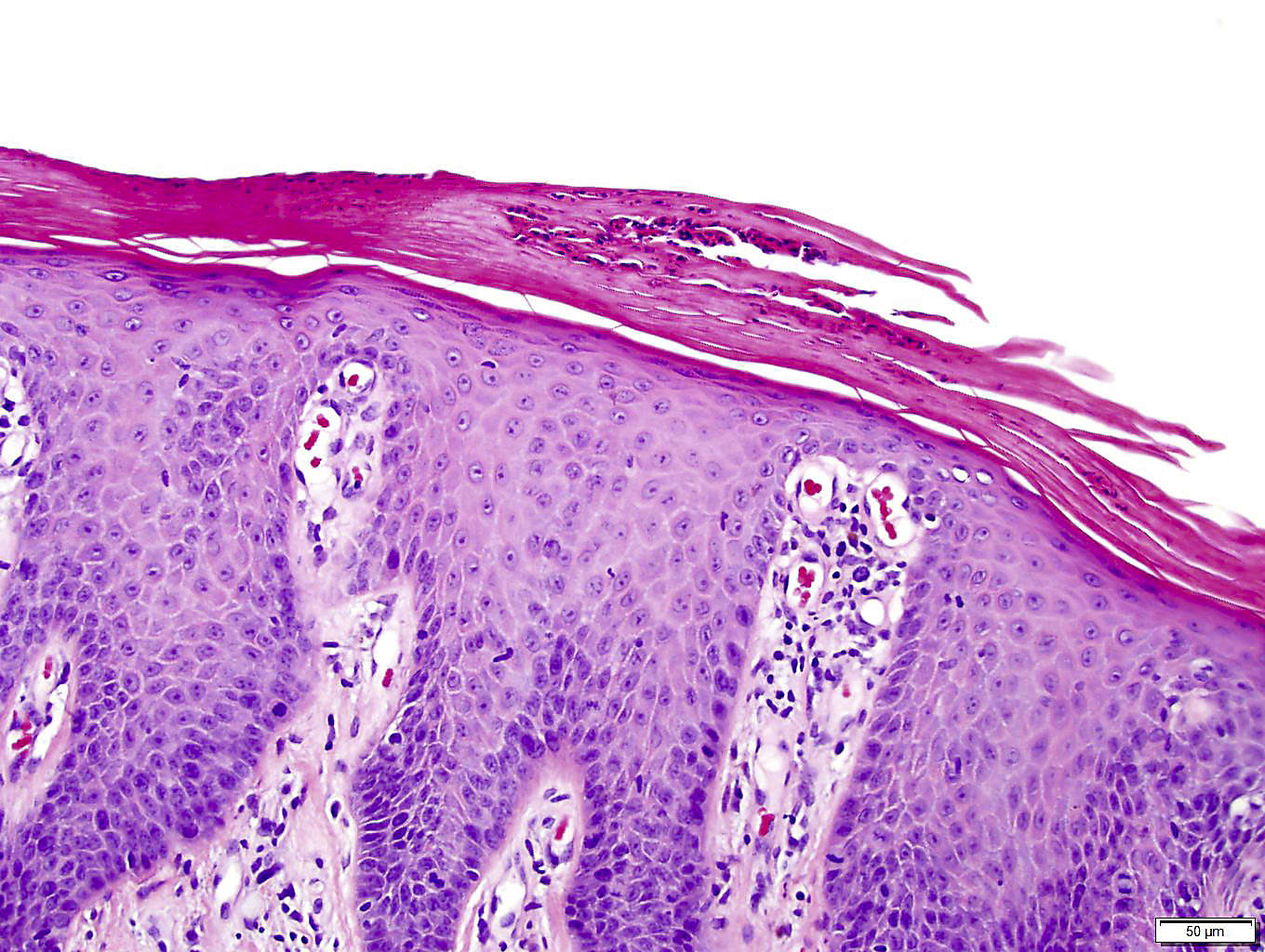
Psoriasis is an autoimmune disease that leads to red, inflamed patches on the skin, which are typically dry, itchy, and scaly. This buildup of skin cells triggers the immune system, which mistakes skin cells for pathogens, but the mechanistic pathways involved are not well understood.
Effector cells are relatively short-lived cells that are activated in an immune response. In healthy people, most effector skin ILCs are ILC2s (1), one of five groups of ILCs. In humans and mice with psoriasis, ILC3s are present in the skin (2) and decrease in response to treatment (3), which suggests that ILC3s lead to the inflammation characteristic of psoriasis. However, it isn’t yet known how or when the composition of ILC2s and ILC3s changes during the course of psoriasis progression. In a study led by Piotr Bielecki, PhD, then in the lab of Richard A. Flavell, PhD, at Yale University School of Medicine, scientists used Chromium Single Cell Gene Expression and Single Cell ATAC (Assay for Transposase Accessible Chromatin) to discover that skin ILCs exist not in discrete states, but along a continuum—a pathogenic-leaning, flexible cell state that may be fully activated by environmental stressors or other cues (4). This has implications for both how immunologists study psoriasis and how they determine what cells or processes may be therapeutically targeted.
Unveiling ILC heterogeneity with single cell transcriptomics
Using a mouse model of psoriasis, Dr. Bielecki and team performed single cell RNA-sequencing (scRNA-seq) on ILCs isolated from ear skin cells during various stages of disease progression. They discovered that cells spanned a transcriptional continuum that reflects a fluid state, including a quiescent-like state and an ILC2 effector state.
Employing a data model to capture different time points during disease development, they were able to describe major types of cells based on their gene expression programs. They labeled three of these states 1) “quiescent-like,” where gene activity represented resting, naïve, and central memory T cells; 2) “ILC2,” with genes associated with ILC2 and T helper 2 cells as well as chemokine genes; and 3) “ILC3-like,” a time-dependent gene expression program that was increased for proinflammatory, ILC3 and T helper 17 cell–associated genes.
As the disease progressed in their mouse model, they saw the continuum shifted to encompass a subset of ILC3-like cells that also expressed cytokines characteristic of ILC2s. More data analysis allowed them to infer that these ILC3-like cells were able to arise from multiple cell states, including transitioning from quiescent cells, ILC2s, and other skin ILCs.
Chromatin accessibility profiling confirms ILC3-like potential
To test whether chromatin shows transitional propensity before psoriasis begins, they performed single cell ATAC-seq on ILCs from naive and interleukin (IL)-23-induced (day 4) mice. (IL-23 stimulates ILC3s to produce cytokines IL-17 and IL-22, which leads to the skin inflammation seen in psoriasis.) Similar to results from their scRNA-seq analysis, gene activity based on chromatin accessibility showed ILC2-specific genes to be active in cells both before and after induction, whereas ILC3-specific genes were more active after induction. In contrast, they found that binding sites for transcription factors associated with ILC2-biased and quiescent-like skin ILCs were accessible in cells before induction and increased as psoriasis was stimulated, allowing these cells the possibility of ILC3-like responses.
Flexible cell state of ILCs points to a new model of psoriasis
Using a mouse model of psoriasis, Dr. Bielecki and his team were able to leverage single cell technologies to characterize skin ILCs and their potential for transition into ILC3s during disease progression. Knowing that these cells live in a state of continuum and should no longer be treated as discrete cell types—as well as that such a flexible cell state might increase the skin’s resilience—the team says will go a long way toward improving how scientists design studies of psoriasis in the future.
References:
- Spencer SP, et al. Adaptation of innate lymphoid cells to a micronutrient deficiency promotes type 2 barrier immunity. Science 343: 432–437 (2014). doi: 10.1126/science.1247606
- Teunissen MBM, et al. Composition of innate lymphoid cell subsets in the human skin: enrichment of NCR+ ILC3 in lesional skin and blood of psoriasis patients. J Invest Dermatol 134: 2351–2360 (2014). doi: 10.1038/jid.2014.146
- Villanova F, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. J Invest Dermatol 134: 984–991 (2014). doi: 10.1038/jid.2013.477
- Bielecki P, et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592: 128–132 (2021). doi: 10.1038/s41586-021-03188-w
