What’s the immunology behind your seasonal flu vaccine?
Single cell insights into the immunogenic response to the seasonal flu vaccine are pointing researchers to more effective vaccine formulations. Follow the path of a flu vaccine and explore recent findings that suggest robust, naïve B-cell responses in lymphoid germinal centers are the key to producing a more diverse antibody repertoire against circulating and emerging influenza virus strains.
While our global attention and public health resources are rightfully set on SARS-CoV-2 and the effort to optimize vaccine treatments against it, other infectious diseases continue to pose a significant health threat. According to the CDC, influenza viruses in the 2019–2020 flu season affected 38 million people in the US alone, with 18 million visits to a health care provider for flu, 400,000 hospitalizations, and 22,000 flu-related deaths. This includes 434 deaths among children under 18 years of age.
The flu is more than an uncomfortable nuisance, but rather, a persistent and potentially deadly infectious disease that warrants revisiting. Annual flu shots are one of our most effective defenses against the flu. One class of vaccines works by exposing the body to small amounts of inactivated virus—that is, virus that has been killed by a chemical, like formaldehyde. This vaccine typically contains a mix of four different viruses, including influenza A (H1N1) virus, an influenza A (H3N2) virus, and two influenza B viruses. Exposure to these inactivated viruses then results in an antibody response that can protect us from contracting the flu. Antibody protection is thought to persist for about 6 months. This waning immunity, along with genetic mutations in circulating influenza viruses, is why we get a new vaccine each year; and it is why scientists and manufacturers regularly revisit and update influenza vaccine formulations to ensure effective immunization against current and emerging strains.
Cell migration and crosstalk: the basics of vaccination
How does the flu vaccine drive an immune response? What are the features of a more robust, long-lasting immune response that can better protect us from the flu? These are questions that scientists ask when they revisit flu vaccine formulations and efficacy. Recently, a cross-functional team of researchers and bioinformaticians from the Washington University School of Medicine, Yale University, and The Scripps Research Institute took up these questions. Specifically, they considered what cells are impacted by the initial vaccine injection, where those cells subsequently travel in the body, and how they can optimally elicit an immune response.
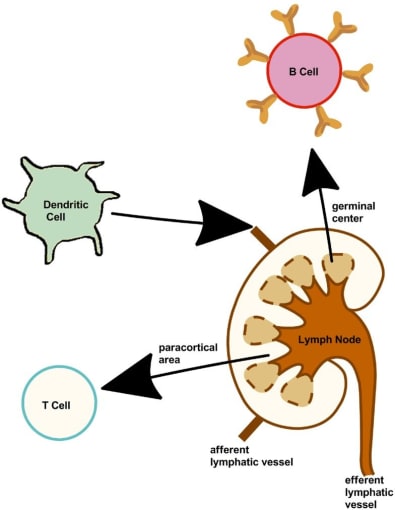
The flu vaccine is typically administered in the deltoid muscle of the upper arm (1). The muscle represents an appropriate processing ecosystem for vaccines as it contains phagocytic and antigen-presenting cells that can take up influenza antigen. It is also streaked with vasculature that can subsequently mobilize the immune response (2). Additionally, the muscle does not contain cells with the necessary receptors for the virus to establish an infection; this, along with the fact that the virus is inactivated, confirms that the recipient cannot contract the flu from the vaccine.
After initial interaction with the antigen, immune cells drain into the lateral axillary lymph nodes via the afferent lymphatic vessels (3). There, the adaptive immune response is further shaped as those antigen-presenting cells activate T cells, which in turn activate naïve B cells—cells that have not yet been exposed to antigen—in the germinal centers of the lymph nodes, where they continue to diversify and mature. The immune response is amplified to the periphery as antigen-specific lymphocytes, including antibody-secreting plasmablasts and T cells, leave the lymph node via the efferent lymphatic vessels.
From injection to antibody protection: the role of germinal center B cells
In order to follow this immunogenic pathway and its evolution over the course of immunization, the research team took serial blood samples and ultrasound-guided fine needle aspiration (FNA) measurements of draining lymph nodes from 8 healthy subjects enrolled in a 2018–2019 influenza vaccination study. Samples were collected at 1, 2, 4, and 9 weeks after vaccination, and studied with particular attention to the development and emergence of a germinal center B-cell immune response, which had not previously been shown to be elicited through influenza vaccination. Using a combination of ELISpot, flow cytometry, and single cell RNA-sequencing (scRNA-seq) analysis, the team identified the cellular components of the samples, including naïve B cells, activated B cells, resting memory B cells, antibody-secreting plasmablast populations, and germinal center B cells (which were present only in the FNA samples from the lymph nodes).
They next analyzed the clonal dynamics of vaccine-responding B-cell clones, contrasting plasmablast and germinal center B-cell clones. While plasmablasts are typically thought to be the drivers behind vaccination success, the researchers found that B cells that participated in the early plasmablast response had high levels of somatic hypermutation (SHM), indicating they were likely memory B cells generated from previous influenza exposure. In contrast, germinal center B cells that were not found in the early blood plasmablast response carried significantly lower mutational burdens, suggesting a predominantly naïve B-cell origin. This confirmed that the contemporary influenza vaccine elicited a germinal center reaction that recruited new B-cell clones to defend against the virus. Moreover, they identified influenza-binding monoclonal antibodies generated from germinal center B-cell clones via ELISA, again suggesting that these clones could effectively target viral epitopes.
In an effort to understand the full binding breadth of monoclonal antibodies derived from plasmablasts and germinal center B-cell clones, the researchers leveraged influenza virus protein microarrays to show that both groups recruited broadly cross-reactive and strain-specific clones. Of all influenza-binding clones, however, germinal center B-cell clones showed a higher proportion of strain-specific monoclonal antibodies compared to the early plasmablast response, suggesting these clones were originally activated by—and potentially more effective binders to—the influenza antigens from the specific virus strains included in the vaccine. Using negative-stain electron microscopy, they mapped the epitopes targeted by some of these germinal center clones, revealing two monoclonal antibodies that targeted unique influenza epitopes. In vivo mouse studies showed that these antibodies were protective against lethal influenza challenge, offering another clue to the key role of germinal center B-cell activation in the immune response to the flu (1).
Optimizing vaccine formulations against the flu
The immunogenic pathway of a flu vaccine contains crucial information about vaccine efficacy, including what cells are involved in the immune response to the vaccine, from where they are derived, and how they can protect us against the virus. As this study demonstrates, scientists are still uncovering the complex cellular dynamics of this immune response with the help of innovative tools, including single cell technology. The evidence suggests that peripheral immunity is quickly ramped up with broadly cross-reactive plasmablasts, recalled from earlier exposure to influenza. However, it is the germinal center B-cell response that enables our bodies to robustly protect against novel influenza strains, unleashing naïve B cells that produce antibodies against novel influenza epitopes. Consequently, new vaccine formulations that promote germinal center reactions may elicit a more diverse catalog of protective antibodies that can sustain a more powerful and long-lasting immune defense against this enduring, seasonal virus.
To find out more about the single cell RNA-sequencing tools this research team used, explore these resources.
References
- JS Turner et al., Human germinal centres engage memory and naive B cells after influenza vaccination. Nature. 586, 127–132 (2020).
- J Zuckerman. The importance of injecting vaccines into muscle. BMJ. 321, 1237–1238 (2000).
- MR Neeland et al., The Lymphatic Immune Response Induced by the Adjuvant AS01: A Comparison of Intramuscular and Subcutaneous Immunization Routes. J. Immunol. 197, 2704–2714 (2016).
