Single cell analysis reveals an unexpected role of immune signaling in neuropsychiatric disorders
Leveraging single cell sequencing, scientists recently discovered that neural interleukin 17a signaling driven by meningeal γδ17 T cells is correlated with anxiety-like behavior in mice. This finding uncovers new insights into complex neuroimmune interactions, and points to immune signaling molecules as possible therapeutic targets for anxiety disorders. Explore further applications of single cell and spatial technology to the study of complex neuropsychiatric disorders and neurodegenerative diseases at our upcoming Global Neuroscience Virtual Symposium.
While the ability to probe the genome has uncovered quite a few of its secrets—especially when it comes to monogenic diseases—there is a lot yet to discover. In the area of psychiatric diseases, this is especially true: not only are most of these disorders polygenic, meaning, the disease is caused by many genetic variants, each having a small contributing effect, but there is also much overlap between major psychiatric disorders in that a genetic variation can confer risk to more than one disease (1).
Of late, single cell sequencing technologies have allowed neuroscience researchers to tease out gene expression changes at single cell resolution—for example, in a recent article highlighting embryonic meningeal development, scientists used single cell RNA-sequencing to study meningeal fibroblast diversity for the first time at that resolution. When it comes to better understanding neuropsychiatric diseases, zoning in on how individual cells act within the brain, as well as with other tissues, will go a long way toward defining previously misunderstood pathways and identifying roles for signaling molecules once thought to be active only in one pathway.
While interleukin 17a (IL-17a) has been widely studied for its role in immunity, new research has found that neuronal IL-17a can have an effect on brain function, specifically learning, memory, and social behavior in autism. In this post, we’ll look at recent research that used single cell analysis to explore possible further roles of immune signaling within the brain, specifically, the meninges, and in the manifestation of anxiety-like behavior (2).
Fitting interleukin 17a into the larger story of neuropsychiatric disorders
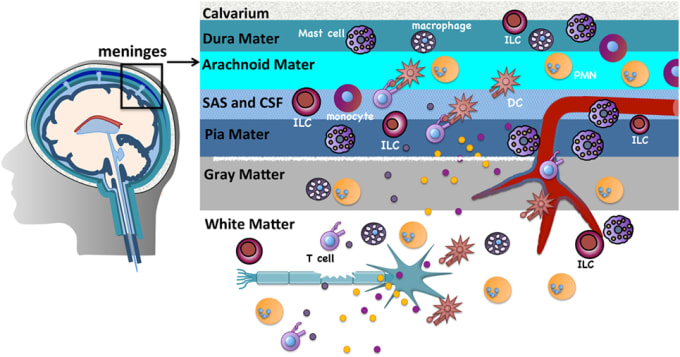
IL-17a is an immune system signaling molecule produced by T helper cells that promotes an immune response to a pathogen. Because it is involved in immune regulatory functions, IL-17a inhibitors may be possible treatments for autoimmune diseases such as rheumatoid arthritis, psoriasis, and inflammatory bowel disease. Notably, though, IL-17a is made throughout the body, including in the meninges—the three-layered covering of the brain and spinal cord—by meningeal γδ17 T cells.
There is growing interest in the role that IL-17a signaling plays in neuropsychiatric disorders such as anxiety, depression, and autism. In mouse models of autism, for instance, researchers showed that IL-17Ra (one type of IL-17a receptor) signaling can promote both decreased and increased sociability. While meningeal immune cells have also been shown to affect brain functions, there is much we don’t know about what is happening at the single cell level. Interleukin 17a may, in fact, be part of a larger story when it comes to brain diseases.
In order to discover more about the molecular diversity and transcriptional landscape of meningeal γδ17 T cells, scientists led by Dr. Kalil Alves de Lima at Washington University in St. Louis used single cell RNA-sequencing to profile γδ T cells from the dural meninges and spleen of 7-day-old or 8-week-old adult mice. Analyzing gene expression readouts, they found high expression of chemokine receptor CXCR6 on meningeal γδ17 T cells, which suggests to them that the CXCR6–CXCL16 axis plays a role in recruitment, retention, and/or activation of γδ17 T cells in the dural meninges.
Most interestingly, they found IL-17a released by meningeal γδ17 T cells to be correlated with anxiety-like behavior in mice through neuronal IL-17Ra signaling (not by way of any other tissue). Using molecular biology techniques, they found that by turning off neuronal IL-17a signaling, anxiety-like behavior in mice lessened. Previous research by another lab found IL-17Ra in cortical neurons in a mouse model of maternal immune activation. The medial prefrontal cortex is known to control responses to threatening stimuli; this study may point to immune molecules in the brain as possible therapeutic targets for mental health disorders like anxiety, where there is a heightened response to what might typically be considered a non-threatening stimulus.
As scientists learn more about the genomic basis of mental health disorders, including how genes and proteins are interacting at the cellular level in specific tissues, interesting links are being discovered between mental health and a variety of pathways, including the immune system. Given the growing interest in the involvement of IL-17a in neuropsychiatric conditions, these findings may spotlight an improved understanding of pathways at the single cell level, supporting further basic and translational research in the search for new therapeutic targets.
We invite you to learn more about how scientists are using single cell and spatial technology from 10x Genomics to translate the complex biology of neuropsychiatric disorders into actionable insights and therapeutic targets. Join us at the Global Neuroscience Virtual Symposium to reimagine neuroscience together. Find a full list of speakers and presentations here.
References
- MR Hoehe and DJ Morris-Rosendahl. The role of genetics and genomics in clinical psychiatry. Dialogues Clin. Neurosci. 20(3), 169–177 (2018).
- K Alves de Lima et al., Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nat. Immunol. 21(11), 1421–1429 (2020).
