Maximize insights, maximize success in pharma
Transforming drug development with single cell and spatial
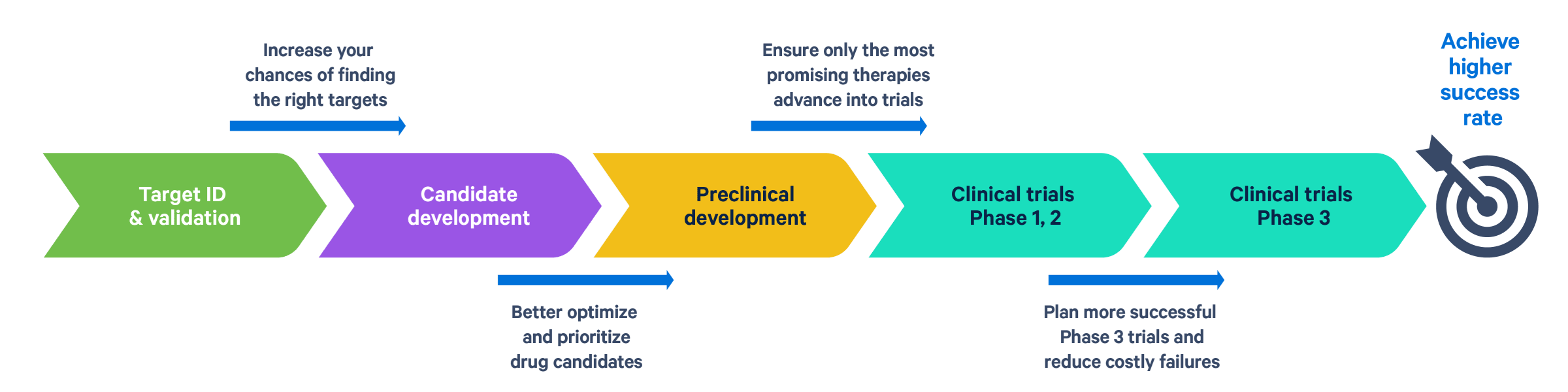
Unlocking the right targets for tomorrow's therapies
Pain point
Challenges selecting the best targets
Traditional tools provide an incomplete understanding of disease biology, making it difficult to separate good targets from bad ones.
How single cell and spatial multiomics can help
Uncover hidden biology for better target selection
Identify and validate actionable targets with a deeper understanding of the cellular heterogeneity and context driving disease pathology.
We're leveraging [10x Genomics technology] in target identification to better understand diseases and for finding new drug targets. We get this really amazing view into things with high resolution, which allows us to understand the differences between the cells of various different diseases we are interested in.Sophia Wild, PhDPrincipal Scientist at Novartis
Single cell and spatial applications in target ID and validation

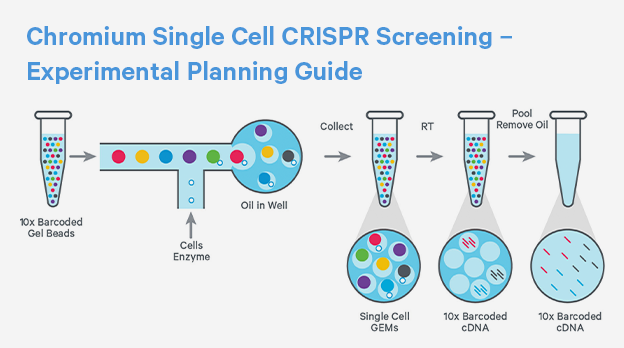
Functional genomics
Identify and validate druggable targets with single cell CRISPR screening
Disease pathology
Profile the heterogeneity and rare cell types implicated in disease progression and response
Biomarker discovery
Identify/validate biomarkers that predict desired disease response to therapeutic interventions
Epigenetic discovery
Pinpoint the epigenetic modifications that drive disease progression
AI-powered discovery
Combine multimodal data with AI to discover new targets and biomarkers
Target ID & validation publications
Publications
Highlighted how disease-specific niches drive pathology, offering actionable insights for druggable targets.
Determined that targets with cell-type-specific expression identified by scRNA-seq were more likely to move into clinical development and pass Phase 1.
Other featured resources
More mechanistic insights, more effective lead prioritization
Pain point
Struggle to refine selection of candidates
Inefficient hit screening tools fail to resolve therapeutic effects and mechanisms with the necessary level of detail.
How single cell and spatial multiomics can help
Reveal therapeutic mechanisms to prioritize the best leads
Assess the MOA of lead candidates at the cellular level on diverse cell populations and pathways.
Our results demonstrate that scBCR-seq can be used for rapid discovery of large, diverse panels of high-affinity antigen-specific antibodies with natively paired heavy- and light-chains when combined with high-quality antigen-specific B-cell sorting.Goldstein LD, et al. Commun Biol. 2: 304 (2019).Quote and image used under CC BY 4.0View publication
Single cell and spatial applications in candidate development

Hit discovery and screening
Identify initial therapeutic candidates, including antibodies, that show potential activity against a desired disease target

Hit-to-lead optimization
Refine the initial hits, including promising antibodies, to improve their properties, such as potency, selectivity, and pharmacokinetics
Candidate development publications
Publications
Resolved antigen-reactive B-cell lineages and reliably pinpointed high-affinity antibodies, accelerating candidate optimization and validation.
Provided high-dimensional insights into T-cell states and transcriptional programs induced by knockin constructs, enabling precise functional validation and refinement of promising candidates.
Other featured resources
Optimize IND readiness with deeper preclinical insights
Pain point
Gathering only limited insights into MOA, pharmacodynamics, pharmacokinetics, toxicity, and safety
Lack of robust molecular data leads to poor predictions about ADME/tox, efficacy, and safety of therapies.
How single cell and spatial multiomics can help
Obtain high-resolution preclinical profiles to support IND filings
Get cellular- and tissue-level data detailing mechanisms of efficacy, safety, and action to select better drug–target combinations to advance to trials.

The preclinical team contacted us to do single cell RNA sequencing with the goal of understanding which of the cell types were responsible for generation of interferon response genes. We were able to find gene signatures associated with the treatment with a TLR7 agonist.Emilio Yangüez, PhDSenior Scientist, Roche
Single cell and spatial applications in preclinical development
Mechanism of action studies
Assess the therapeutic MOA using preclinical models

Toxicity and safety
Monitor and evaluate the toxicity and safety of the drug using preclinical models
Pharmacokinetics/pharmacodynamics
Examine the biodistribution and impact of therapeutic compounds on cells and tissues
Immunogenicity assessment
Evaluate the potential of drug candidates to elicit an immune response

Research on efficacy and response
Assess the mechanisms of efficacy and response of the drug in preclinical studies
Preclinical development publications
Publications
Demonstrated the mechanism through which TGFβ-blockade reshapes the tumor microenvironment to enhance immunotherapy efficacy, providing strong preclinical evidence for the effectiveness of this combination strategy.
Uncovered cell-specific transcriptional responses and population shifts in the liver following TCDD treatment, which were critical for understanding the mechanisms of TCDD-induced toxicity.
Revealed a rare neural cell population that may explain the neurotoxicity observed in individuals receiving CD19 CAR-T therapy as a result of unintended targeting.
Highlighted how the spatial organization of cells and their interactions are often crucial for understanding therapeutic responses and provided evidence for using ligand–receptor activity as a predictive biomarker for therapy response.
Other featured resources
Increase clinical trial success with high-resolution insights
Pain point
Imprecise understanding of the biology of therapeutic response and resistance puts trials at risk
Not fully understanding mechanisms dictating response and resistance increases the chance of clinical trial failures.
How single cell and spatial multiomics can help
Gain a more complete view of therapeutic response in clinical research patient populations
Better understand response and resistance, plus confirm mechanisms underlying efficacy, safety/toxicity, and drug action to mitigate late-stage failures.
In what we believe is one of the first clinical trials to incorporate systematic scRNAseq analysis of paired pre- and on-treatment tumor biopsies from all patients, we identified a potential mechanism underlying the cooperativity observed between BRAF/MAPK inhibition and immune response.Tian J, et al. Nat Med. 29 (2023).Quote and image used under CC BY 4.0Read blog about this Phase 2 trial
Single cell and spatial applications in clinical trial exploratory research

Mechanism of action studies
Assess the therapeutic MOA in clinical research samples

Toxicity and safety
Monitor and evaluate the toxicity and safety of the drug in clinical research samples

Pharmacokinetics/pharmacodynamics
Examine the biodistribution and impact of therapeutic compounds on cells and tissues

Immunogenicity assessment
Evaluate the potential of drug candidates to elicit an immune response

Research on efficacy and response
Assess the mechanisms of efficacy and response of the drug in clinical research samples
Clinical trial exploratory research publications
Publications
Demonstrated a strong association between spatial biomarkers and treatment responses.
Determined T-cell clones and dynamics correlated with positive response and showed power of peripheral biomarkers for monitoring.
Characterized tumor microenvironment features that played a critical role in therapeutic success or resistance.
Highlighted the diversity of cells within the TIME and pinpointed the major pathologic response differences seen between treatment regimens.
Revealed how vaccination impacted the immune repertoire post-vaccination and validated the efficacy mechanism of this treatment strategy.
Clarified the transcriptional mechanisms promoting treatment response and potentially alleviating tumor resistance to standard treatments.
Other featured resources
Tools tailored for every stage of drug development
| Platform | |||
| When to use | Comprehensive single cell data Ideal for deep characterization of cell populations and states. | High-resolution spatial gene expression Understand complex tissues, cellular neighborhoods, and cell to cell interactions. Integration with other spatial-omics, histology, and morphology. | |
| Why to use | Unbiased single cell discovery High per-gene sensitivity | Unbiased spatial discovery | Targeted spatial exploration High per-gene sensitivity |
| Analytes | Whole transcriptome gene expression Protein TCR, BCR CRISPR perturbations Chromatin accessibility | Whole transcriptome gene expression Human immuno-oncology protein panel H&E morphology Protein immunofluorescence | Targeted gene expression (up to 5,000 genes) Custom panels (unique gene markers, expression signatures, isoforms, gene fusions, and more) H&E morphology Protein immunofluorescence |
| Resolution | Single cell | Transcripts assigned to 2-µm areas | Single cell |
| Data readout | NGS-based | NGS-based | Imaging-based |
| Sample compatibility | Cell and nuclei suspension, Tissue (fresh, frozen, FFPE, PFA-fixed) Flow-sorted cells Fixed whole blood and PBMCs Organoids | FFPE tissue Fresh frozen tissue Fixed frozen tissue | FFPE tissue Fresh frozen tissue |

Access the power of single cell and spatial through Service Providers
Looking for flexibility or additional support? Our Service Provider Network offers a way to access 10x Genomics’ cutting-edge technologies without the need for an in-house setup.